![[US Patent & Trademark Office, Patent Full Text and Image Database]](United States Patent 4,152,215.files/patfthdr.gif)
| United States Patent |
4,152,215 |
| Yoshino , et al. |
May 1, 1979 |
Apparatus for controlling pH of culture solution for a living
organism
Abstract
Electrolysis is employed for raising or reducing pH of culture solution.
Electrolyte contained in a vessel having at least one ion exchange membrane as a
wall is provided so as to confront the culture solution, with the ion exchange
membrane lying therebetween. Electrodes are provided both in the electrolyte and
the culture solution. With these electrodes the electrolysis is carried out
through the ion exchange membrane. Thus formed alkali or acid changes the pH of
the culture solution. By controlling the time or supplied current for the
electrolysis, required pH can be accurately obtained.
| Inventors: |
Yoshino; Yohzoh (Hirakata, JP);
Kawabe; Hidehiko (Moriguchi, JP) |
| Assignee: |
Matsushita Electric Industrial Co., Ltd.
(Osaka, JP) |
| Appl. No.: |
851583 |
| Filed: |
November 14, 1977 |
Foreign Application Priority Data
|
Nov 12, 1976[JP] |
51-136652 |
| Current U.S. Class: |
435/286.1; 47/62N; 47/62R;
435/287.1 |
| Intern'l Class: |
C12K 001/10 |
| Field of Search: |
195/127 204/180 P,301 47/62
|
References Cited [Referenced
By]
U.S. Patent Documents
| 2739934 |
Mar., 1956 |
Kunin |
204/180. |
| 3671412 |
Jun., 1972 |
Lohr |
204/301. |
| 3989613 |
Nov., 1976 |
Gritzner |
204/180. |
| 4043895 |
Aug., 1977 |
Gritzner |
204/301. |
| 4049519 |
Sep., 1977 |
Sloan |
204/301. |
| 4057483 |
Nov., 1977 |
Giuffrida |
204/301. |
| 4070263 |
Jan., 1978 |
Treille et al. |
204/301. |
Primary
Examiner: Jones; Raymond N.
Assistant Examiner: Warden; Robert J.
Attorney, Agent or Firm: Wenderoth, Lind & Ponack
Claims
What we claim is:
1. An apparatus for controlling the pH of a
culture solution for a living organism, which comprises: a culture solution
vessel containing a culture solution; a pH electrode immersed in the culture
solution for measuring the pH of the culture solution and for producing a signal
in accordance with the pH measured; an electrolyte vessel immersed in the
culture solution, the electrolyte vessel containing electrolyte therein and at
least a portion of the wall of the electrolyte vessel being composed of an ion
exchange membrane so that said ion exchange membrane is in contact with the
culture solution; a first electrolysis electrode immersed in said electrolyte; a
second electrolysis electrode immersed in the culture solution in a manner such
that said ion exchange membrane is positioned between said first and second
electrolysis electrodes; a DC voltage applying means for applying a DC voltage
between said first and second electrolysis electrodes; and a pH controller that
activates the DC voltage applying means in accordance with the signal produced
by the pH electrode.
2. An apparatus according to claim 1, wherein a
further portion of the wall of the electrolyte vessel is composed of a second
ion exchange membrane, and a third electrolysis electrode is immersed in the
culture solution in a manner such that said second ion exchange membrane is
positioned between said first and third electrolysis electrodes.
3. An
apparatus according to claim 1, further comprising: a second electrolyte vessel
which contains electrolyte, a portion of the wall of said second electrolyte
vessel being composed of an anion exchange membrane; a third electrolysis
electrode immersed in the electrolyte contained in said second electrolyte
vessel; a fourth electrolysis electrode immersed in the culture solution in a
manner such that said anion exchange membrane is positioned between said third
and fourth electrolysis electrodes; and wherein said ion exchange membrane is
composed of a cation exchange membrane, said first and second electrolysis
electrodes are respectively used as an anode and a cathode, and wherein said
third and fourth electrolysis electrodes are respectively used as a cathode and
an anode.
4. An apparatus according to claim 1, wherein said culture
solution is circulated so that eduction of insoluble matters on said ion
exchange membrane and on said second electrolysis electrode is avoided.
Description
BACKGROUND OF THE INVENTION
The present invention relates to a
method of and an apparatus for controlling the pH of culture solution for a
living organism.
It is quite important to control pH in the culture of a
living organism such as in hydroponics. Such method has hitherto been used as
neutralizing by adding alkali or acid into a culture solution tank from storage
tanks. The amount of alkali or acid to be added is based on the measurement with
a pH meter. When automatic control is required, the storage tanks for alkali and
acid are provided with electrovalves which control the release of the contents,
and the electrovalves are driven by a controller which works responsively to the
signal from the pH meter having an electrode inserted into the culture solution.
Such method, however, has not been satisfactory for reasons as follows:
A desirable value of a pH of the culture solution is in a narrow range
near neutrality. Therefore when concentrated alkali and acid is used, it is
difficult to maintain pH in the desirable range because pouring thereof easily
causes large change of pH. To accurately control a pH, it is desirable to use
dilute alkali and acid. The use of dilute alkali and acid requires large tanks
for storing them. Further it is necessary to supplement alkali or acid in the
storage tanks frequently. Further the electrovalves for the tanks must be
corrosion-resistant because alkali or acid flows therethrough. Further, the case
possibly occurs that alkali solution reacts with CO.sub.2 gas in air to make
carbonate, and it enters into the culture solution through neutralization,
resulting in bad effect on plants, or it chokes up the gateway by solidifying.
SUMMARY OF THE INVENTION
It is an object of the invention to
provide a method of controlling the pH of culture solution whereby such control
can be performed at high accuracy.
It is a further object of the
invention to provide an apparatus for such pH-control having a simple and
compact construction.
According to the present invention, electrolysis
through an ion exchange membrane is employed. A vessel having a partition wall
of an ion exchange membrane is provided in the manner such that the culture
solution abuts on the ion exchange membrane from outside of the vessel. Within
the vessel is held electrolyte of inorganic ions. Electrodes are provided on
both sides of the ion exchange membrane, i.e. both in the culture solution and
the electrolyte. With these electrodes electrolysis is performed through the ion
exchange membrane.
As the result of the electrolysis, formed alkali or
acid changes a pH of the culture solution. By controlling the time and supplied
current of the electrolysis, required pH can easily be obtained.
BRIEF
DESCRIPTION OF THE DRAWINGS
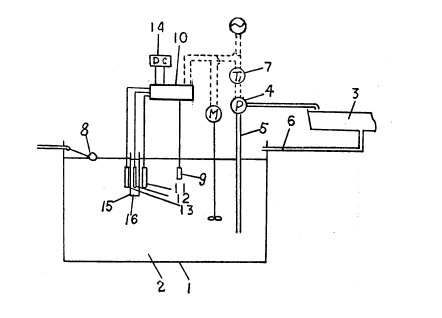
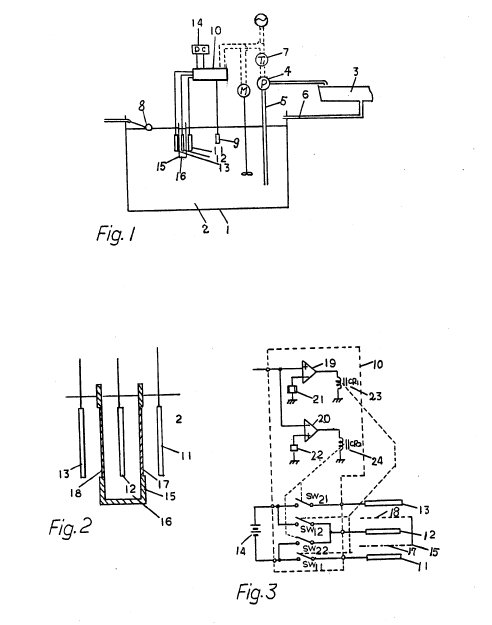
FIG. 1 is a schematic sectional view for
explaining an automatic pH controlling method for culture solution embodying the
present invention;
FIG. 2 is a sectional view illustrating an important
part of a pH controlling apparatus illustrated in FIG. 1;
FIG. 3 is a
circuit diagram of the pH controlling apparatus illustrated in FIG. 1;
FIGS. 4 and 5 are sectional views illustrating other embodiments of such
part of the pH controlling apparatus as illustrated in FIG. 2;
FIG. 6 is
a schematic diagram illustrating another embodiment of the invention;
FIG. 7 is an elevational view partly in section of a part of the
apparatus illustrated in FIG. 6.
DETAILED DESCRIPTION OF THE INVENTION
FIG. 1 illustrates a pH controlling apparatus which is used in a
hydroponics system. A culture solution tank 1 is provided for storing culture
solution 2. The culture solution 2 is conveyed to a growing vessel 3 by a pump
4. It is pumped up through a supply pipe 5 and returns from the growing vessel 3
to the culture solution tank 1 through a drain pipe 6. The operation of the pump
4 is controlled by a timer 7 so as to be driven intermittently. As the case may
be the pump 4 is driven continuously. The level of the culture solution is
maintained constant by a constant-level regulator 8. The pH of the culture
solution 2 is measured by a pH electrode 9 and obtained data signal is
transmitted to a controller 10. The controller 10 is connected with electrodes
11, 12 and 13 for electrolysis and a DC power source 14, and controls the
supplying of current from the DC power source 14 to the electrodes 11, 12 and
13. Such control is made in accordance with the result of collation between the
pH measured by the pH electrode 9 and a required range of pH preset in the
controller 10. When the measured value is out of the preset range, the DC
current from the DC power source 14 is supplied between the electrodes 11 and 12
or the electrodes 12 and 13, whereby the electrolysis occurs so as to maintain
the pH of culture solution 2 in the preset range. The electrode 12 is placed
within an electrolyzing vessel 15 in which an electrolyte 16 is held.
As
illustrated in FIG. 2, the electrolyzing vessel 15 is box-shaped and two opposed
walls thereof are partially constructed by ion exchange membranes 17 and 18. In
this embodiment, the membrane 17 is a cation exchange membrane and the membrane
18 an anion exchange membrane. The electrodes 11 and 12 confront each other,
with the cation exchange membrane 17 lying therebetween. And the electrodes 12
and 13 confront each other, with the anion exchange membrane 18 lying
therebetween. As the electrolyte 16, for example, K.sub.2 SO.sub.4 aqueous
solution is used. The electrodes 11, 12 and 13 may be of platinum.
The
controller is, for example, constructed as illustrated in FIG. 3. A pH signal
voltage from the pH electrode 9 is supplied to the comparators 19 and 20.
Reference voltages for the comparators 19 and 20 are supplied from variable
voltage sources 21 and 22, respectively. The variable voltage source 21 is
preset correspondingly to the lower limit of the required pH range and the
variable voltage source 22 to the upper limit. The coupling between the
comparators 19 and 20 and the input signals are in such relation that the
comparator 19 generates an output signal if the pH signal voltage is lower than
the reference voltage of the variable voltage source 21 and the comparator 20
generates an output signal if the pH signal voltage is higher than the reference
voltage of the variable voltage source 22. The outputs of the comparators 19 and
20 are coupled to relays CR.sub.1 23 and CR.sub.2 24, respectively. The
electrode 11 is connected with the negative terminal of the DC power source 14
through a contact SW.sub.11 which is one of the contacts of the relay CR.sub.1
23. The electrode 13 is connected with the positive terminal of the DC power
source 14 through a contact SW.sub.21 which is one of the contacts of the relay
CR.sub.2 24. The electrode 12 is connected with the positive terminal of the DC
power source 14 through another contact SW.sub.12 of the relay CR.sub.1 23, and
with the negative terminal of the DC power source 14 through another contact
SW.sub.22 of the relay CR.sub.2 24.
Thus when the pH signal voltage is
lower than the preset range, i.e. lower than the reference voltage of the
variable voltage source 21, the relay CR.sub.1 23 is driven by the output of the
comparator 19 and the contacts SW.sub.11 and SW.sub.12 are made. Thereby
electrolysis through the cation exchange membrane 17 is carried out by the
current supplied from the DC power source 14, with the electrode 12 as the anode
and the electrode 11 as the cathode.
The reaction in the electrolyzing
vessel 15 is represented as follows:
2K.sup.+ +SO.sub.4.sup.2- +2H.sub.2
O.fwdarw.1/20.sub.2 .uparw.+2H.sup.+ +SO.sub.4.sup.2- +2e
The K.sup.+
ions are transferred by migration to the side of the electrode 11 through the
cation exchange membrane 17.
On the other hand, the reaction in the
culture solution 2 is as follows:
2H.sub.2 O+2e.fwdarw.H.sub.2
.uparw.+2OH.sup.- +2K.sup.+
the OH.sup.- ions thus generated on the
electrode 11 raise the pH of the culture solution 2. The electro neutrality in
the culture solution 2 is maintained by the K.sup.+ ions transferred from the
electrolyte 16 by the amount corresponding to electrical quantity for the
electrolysis.
Thus the pH of the culture solution is raised to within
the required range.
When the pH signal voltage is higher than the preset
range, i.e. higher than the reference voltage of the variable voltage source 22,
the relay CR.sub.2 24 is driven by the output of the comparator 20 and the
contacts SW.sub.21 and SW.sub.22 are made. Thereby electrolysis through the
anion exchange membrane 18 is carried out, with the electrode 13 as the anode
and the electrode 12 as the cathode.
The reactions thereof are as
follows:
Anode: 2H.sub.2 O.fwdarw.2H.sup.+ +SO.sub.4.sup.2- +1/20.sub.2
.uparw.+2e
Cathode: 2H.sub.2 O+2K.sup.+ +SO.sub.4.sup.2-
+2e.fwdarw.H.sub.2 .uparw.+2OH.sup.- +2K.sup.+
thus in the culture
solution 2 wherein the anodic reaction occurs, the concentration of H.sup.+ ion
increases accordingly as the electrolysis progresses, and the pH falls.
Although K.sub.2 SO.sub.4 aqueous solution is used as the electrolyte 16
in the above-mentioned embodiment, other material may be used. It is preferable
that the electrolyte 16 not have a bad effect on plants because the ions within
the electrolyzing vessel 15 are transferred to the culture solution 2 through
the ion exchange membranes 17 and 18. Examples of satisfactory materials result
from combination between K.sup.+, NH.sub.4.sup.+, Ca.sup.++, Mg.sup.++,
NO.sub.3.sup.-, SO.sub.4.sup.2- and PO.sub.4.sup.3- which are the ions contained
in the culture solution 2 in substantial amounts.
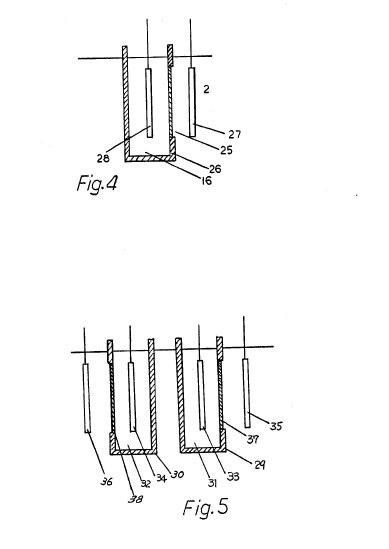
The electrolysis may
be carried out by the use of two electrodes and one ion exchange membrane. As
illustrated in FIG. 4, for example, an anion exchange membrane 25 is employed
and formed as one partition wall of an electrolyzing vessel 26. Electrodes 27
and 28 confront each other, with the anion exchange membrane 25 lying
therebetween.
If the pH of the culture solution should be raised, the
electrode 28 in the vessel 26 is used as an anode and the electrode 27 as an
cathode. The reactions in such case are as follows: ##STR1## where the X.sup.-
and M.sup.+ represent ions contained in the culture solution 2, as fertilizer
such as K.sup.+, NH.sub.4.sup.+, NO.sub.3.sup.-, etc. These ions may be a multi
valent ion such as Ca.sup.++, Mg.sup.++, PO.sub.4.sup.3-, SO.sub.4.sup.2-, etc..
If the pH of the culture solution should be lowered, the electrodes 27
and 28 are used with polarities the reverse of the above case. The reactions are
as follows:
Cathode: 2H.sub.2 O+K.sup.+ +SO.sub.4.sup.2-
+2e.fwdarw.H.sub.2 +2OH.sup.- +K.sup.+
anode: H.sub.2 O+2M.sup.+
+2X.sup.- .fwdarw.1/2O.sub.2 +2H.sup.+ +2X.sup.- +SO.sub.4.sup.2-
instead of the anion exchange membrane 25, a cation exchange membrane
can be used for constructing the system in the similar manner.
Furthermore, pH of the culture solution may be also controlled by an
apparatus as shown in FIG. 5. This apparatus has two electrolyzing vessels 29
and 30 which respectively contain electrolyte 31 and electrolyte 32, two
electrodes 33 and 34 which are respectively immersed in the electrolyte 31 and
electrolyte 32, and two electrodes 35 and 36 immersed in the culture solution 2.
This apparatus is particularly available for the case where ion-species of
cations and anions, which respectively migrate from electrolyte 31 and
electrolyte 32 to the culture solution 2, have to be restricted for some reason
and the compounds composed of these ions show poor solubility. For example, if
cations are to be restricted to Ca.sup.++ and anions to SO.sub.4.sup.2-,
CaSO.sub.4 is produced as a compound of these ions. This compound shows poor
solubility of the order of 2g/l and thereby it becomes difficult to allow the
electrolyte to have a sufficient conductivity for causing satisfactory
electrolysis. In this case, where the construction as shown in FIG. 5 is
employed, an exchange membrane 37 provided at a portion of the electrolyzing
vessel 29 is composed of a cation exchange membrane, the electrolyte 31 contains
Ca(NO.sub.3).sub.2 4H.sub.2 O solution with a high solubility, and where the
electrodes 33 and 35 provided on either side of the exchange membrane 37 are
respectively used as an anode and a cathode, it is possible to permit Ca.sup.++
cations in the electrolyte 31 to migrate into the culture solution 2 by
electrolysis. On the other hand, where an exchange membrane 38 provided at a
portion of the electrolyzing vessel 30 is composed of anion exchange membrane,
the electrolyte 32 contains K.sub.2 SO.sub.4 solution, and where the electrodes
34 and 36 provided on either side of the exchange membrane 38 are respectively
used as a cathode and an anode, it is possible to permit SO.sub.4.sup.2- anions
in the electrolyte 32 to migrate into the culture solution by electrolysis.
In order to make the culture solution pH controlling apparatus
miniaturize in size and light in weight, it becomes necessary to make the amount
of the electrolyte 16 in the electrolyzing vessel 15 less as compared with that
of the culture solution. However, when pH of the culture solution 2 is rectified
by electrolysis, pH of the electrolyte 16 changes to the adverse direction with
respect to the pH rectifying direction of the culture solution and this pH
change of the electrolyte becomes larger with the decreasing of the amount of
the electrolyte. Therefore, in order to restrict the pH change of the
electrolyte 16 to as small as possible, it is preferable that the electrolyte 33
includes, as components, weak acid or weak base ion with a large buffer
capacity, for example H.sub.2 PO.sub.4.sup.-, HPO.sub.4.sup.2-, Ca.sup.++,
Mg.sup.++ or mixture thereof, among the combinations of a large number of ions
included as fertilizers in the culture solution 2.
Furthermore, in FIGS.
2 and 3, if the ion exchange membrane 17 is composed of an anion exchange
membrane, the ion exchange membrane 18 a cation exchange membrane, and like
operation as described above is performed, namely, if electrolysis is caused by
arranging, as an anode, the electrode 12 in the electrolyzing vessel 15 and, as
a cathode, the electrode 11 in the culture solution 2 in a manner that they are
positioned on either side of anion exchange membrane 17 or by arranging, as an
anode, the electrode 13 in the culture solution 2 and, as a cathode, the
electrode 12 in the electrolyzing vessel 15 in a manner that they are positioned
on either side of the cation exchange membrane 18, ions in the culture solution
2 migrate into the electrolyzing vessel 15 through the ion exchange membranes 17
and 18, and thereby pH of the culture solution 2 may be controlled with
maintenance of electro neutrality of the culture solution 2 and the electrolyte
16. In this case, because components included in the electrolyte 16 are never
carried into the culture solution 2 by the electro-migration, it is unnecessary
to pay attention to whether the components to be added in the electrolyte 16
adversely affect living organisms, and therefore they may be optionally
selected. Thus, it becomes possible to add to the electrolyte 16 neutral salts
with a high equivalent conductivity such as KClO.sub.4, K.sub.4 Fe(CN).sub.6,
K.sub.3 Fe(CN).sub.6, KI or the like, other than the combination of ions
included as fertilizer components in the culture solution 2, and moreover it
becomes also possible to add to the electrolyte 16 materials which give a large
pH-buffer-capacity to the electrolyte, for example the material such as MH.sub.3
(C.sub.2 O.sub.4).sub.2 2H.sub.2 O, C.sub.6 H.sub.4 (COOM)COOH), MH.sub.2
PO.sub.4, M.sub.2 HPO.sub.4, M.sub.2 HPO.sub.4, M.sub.2 B.sub.4 O.sub.7,
MHCO.sub.3, M.sub.2 CO.sub.3 or the mixture thereof wherein M denotes an
alkaline metal such as K, Na, Li, or the like. Furthermore, if there is employed
such a construction that ions emigrate only from the culture solution 2 into the
electrolyzing vessel 15 by electrolysis, it becomes unnecessary to restrict the
electrode reaction on the electrode 12 to water decomposition which generates
H.sup.- and OH.sup.-. Rather, in order to avoid a large pH-variation of the
electrolyte 16, the electrolyte 16 and the electrode 12 may be composed of a
material which permits the reaction which occurs prior to an O.sub.2 evolution
reaction as an anode reaction and prior to H.sub.2 evolution as a cathode
reaction; that is a dissolution-decomposition-reaction of Cu and Ag or a
dissolution-deposition-reaction of Pb, Ni or Sn which occurs, due to a hydrogen
overvoltage, prior to charge-discharge of H.sub.2 ; or an oxidation-reduction
reaction of ions such as
Sn.sup.4+ .revreaction.Sn, Fe(CN).sub.6.sup.3-
.revreaction.Fe(CN).sub.6.sup.4-, Fe.sup.3+ .revreaction.Fe.sup.2+ or
the like. For example, in the event that Cu and CuSO.sub.4 solution are
respectively employed as the electrode 13 and the electrolyte 16, if the
electrode 13 is used as an anode, an anode process becomes as follows:
Cu.fwdarw.Cu.sup.2+ +2e+2X.sup.-
wherein 2X.sup.- emigrates from
the culture solution, and if the electrode 13 is used as a cathode, a cathode
process becomes as follows:
Cu.sup.2+ +SO.sub.4.sup.2+
+2e.fwdarw.Cu.uparw.+SO.sub.4.sup.2- +2M.sup.+
wherein 2M.sup.+
emigrates from the culture solution, whereby pH of the electrolyte in the
electrolyzing vessel 15 substantially does not change.
In such
arrangement, the material of the electrode 12 in the electrolyzing vessel 15
should be selected by considering the composition of the electrolyte 16. Namely,
if it is desired to produce a dissolution-deposition reaction by employing the
electrolyte including a salt such as CuSO.sub.4, AgNO.sub.3, NiCl.sub.2 or
(CH.sub.3 COO).sub.2 Pb, the electrode material should be composed of Cu, Ag, Ni
or Pb and if it is desired to produce an oxidation-reduction reaction of ions or
a water decomposition by employing the electrolyte which includes mixed solution
of SnCl.sub.2 -SnCl.sub.4, FeSO.sub.4 -Fe(SO.sub.4).sub.3 or K.sub.4
Fe(CN).sub.6 -K.sub.3 Fe(CN).sub.6, the electrode material should be composed of
a material being very or relatively stable in a range of 3-10 pH, for example
Pt, Au, Carbon, stainless steel or Ni.
Furthermore, the electrolyzing
apparatus for controlling pH of the culture solution may be set not only in the
culture solution tank 1 as shown in FIG. 1, but also in the culture supplying
tube 5, the growing vessel 3 or the draining tube 6. If the culture solution 2
is circulated, it may be set in any portion other than the portions as described
above.
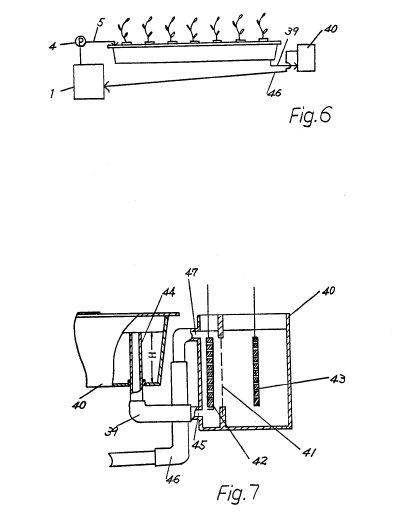
In FIGS. 6 and 7, there is shown a pH controlling system for
controlling pH of the culture solution wherein the electrolyzing device is
placed at the end of a draining tube 39 with the culture solution being
circulated. The electrolyzing vessel 40 of the electrolyzing device has therein
an ion exchange membrane 41 and a pair of electrodes 42 and 43. In this system,
when the depth of the culture solution 2 exceeds the height (H) of a water level
controlling tube 44 for controlling the level of the culture solution to a
constant level, the culture solution overflows into the electrolyzing vessel 40
through the inside of the level controlling tube 44, the draining tube 39 and an
inlet 45 of the electrolyzing vessel 40, which inlet 45 is below the lower edge
of the electrode 42, and thereafter it flows into a tube 46 through a space
between the electrode 42 and the ion exchange membrane 41 and through an outlet
47 of the electrolyzing vessel 40, which outlet 47 is above the upper edge of
the electrode 42. If the outlet 47 is below the upper level of the water level
controlling tube 44, the culture solution in the electrolyzing vessel 40 keeps
its level equal to the upper level of the water level controlling tube 44,
thereby preventing the ion exchange membrane 41 from being damaged due to its
drying. Furthermore, this system is effective in that an eduction of insoluble
matter such as Ca(OH).sub.2, Mg(OH).sub.2 or the like, on the electrode 42 or on
the exchange membrane 41 may be avoided because the culture solution is churned
at the vicinity of the electrode 42 and the exchange membrane 41 by the
circulation of the culture solution, which Ca(OH).sub.2 and Mg(OH).sub.2 is
formed when Ca.sup.++ and Mg.sup.+ + in the culture solution combine with
OH.sup.- produced by electrolysis.
In the system as described above, it
has a capacity to form in the culture solution by the electrolysis for 1 Hr, for
example, an amount of an acid which is nearly equal to that in the case where 36
N-H.sub.2 SO.sub.4 of about 1 ml is added to the culture solution. Since it is
possible to decrease an electric current and to shorten a period of
electrolysis, the electrolyzing apparatus of the present invention is available
for a very accurate pH control and for a pH control which requires a slow pH
change such as for a living organism which should not be subjected to an abrupt
change of pH in a neutralization range of solution.
* * * * *
![[US Patent & Trademark Office, Patent Full Text and Image Database]](United States Patent 4,152,215.files/patfthdr.gif)
![[Home]](United States Patent 4,152,215.files/home.gif)
![[Boolean Search]](United States Patent 4,152,215.files/boolean.gif)
![[Manual Search]](United States Patent 4,152,215.files/manual.gif)
![[Number Search]](United States Patent 4,152,215.files/number.gif)
![[Help]](United States Patent 4,152,215.files/help.gif)
![[Bottom]](United States Patent 4,152,215.files/bottom.gif)
![[View Shopping Cart]](United States Patent 4,152,215.files/cart.gif)
![[Add to Shopping Cart]](United States Patent 4,152,215.files/order.gif)
![[Image]](United States Patent 4,152,215.files/image.gif)
![[US Patent & Trademark Office, Patent Full Text and Image Database]](United States Patent 4,152,215.files/patfthdr.gif)
![[Home]](United States Patent 4,152,215.files/home.gif)
![[Boolean Search]](United States Patent 4,152,215.files/boolean.gif)
![[Manual Search]](United States Patent 4,152,215.files/manual.gif)
![[Number Search]](United States Patent 4,152,215.files/number.gif)
![[Help]](United States Patent 4,152,215.files/help.gif)
![[Bottom]](United States Patent 4,152,215.files/bottom.gif)
![[View Shopping Cart]](United States Patent 4,152,215.files/cart.gif)
![[Add to Shopping Cart]](United States Patent 4,152,215.files/order.gif)
![[Image]](United States Patent 4,152,215.files/image.gif)



