![[US Patent & Trademark Office, Patent Full Text and Image Database]](United States Patent 5,598,663.files/patfthdr.gif)
| United States Patent |
5,598,663 |
| Kikuchi |
February 4, 1997 |
Hydroponic nutrient solution control system
Abstract
A hydroponic nutrient solution control system capable of performing the
nutrient solution control automatically, accurately, effectively, and
efficiently. In the system, the supplies of the acid nutrient ion ingredient
solutions and the alkali nutrient ion ingredient solutions from the acid
solution tanks and the alkali solution tanks to the nutrient solution tank are
controlled according to the pH measured by the pH meter and the ion
concentrations measured by the ion analyzer. The system may also controls the
supplies of the high concentration nutrient solutions and the water from the
high concentration nutrient solution tanks and the water supply according to the
electrolytic conductivity measured by the electrolytic conductivity meter.
| Inventors: |
Kikuchi; Hiroshi (Tokyo-to, JP) |
| Assignee: |
Kabushiki Kaisha Toshiba (Kawasaki, JP)
|
| Appl. No.: |
301039 |
| Filed: |
September 6, 1994 |
Foreign Application Priority Data
|
Dec 12, 1989[JP] |
1-320637 |
| Current U.S. Class: |
47/62N |
| Intern'l Class: |
A01G 031/00 |
| Field of Search: |
47/62 N,1.01 137/1,5
|
References Cited [Referenced
By]
U.S. Patent Documents
| 4149970 |
Apr., 1979 |
Atkins |
47/62. |
| 4152215 |
May., 1979 |
Yoshino |
47/62. |
| 4294037 |
Oct., 1981 |
Mosse |
47/62. |
| 4320594 |
Mar., 1982 |
Raymond |
47/62. |
| 4926585 |
May., 1990 |
Dreschel |
47/62. |
| 4992942 |
Feb., 1991 |
Bauerle |
47/62. |
| 5184420 |
Feb., 1993 |
Papadopoulos |
47/62. |
| Foreign Patent Documents |
| 0110111 |
Jun., 1984 |
EP |
47/62. |
| 7712467 |
May., 1978 |
NL |
47/62. |
| 2192875 |
Jan., 1988 |
GB |
47/62. |
Other References
Oakton WD-00661-10 "Dissolved Solid Tester" Considered
by the examiner to be an ion analyzer means as claimed by the applicant,
Devices of this sort have been in use in the hydroponics industry for many
years.
The ABC of NFT by Dr. Allen Coope pp. 47-89.
|
Primary Examiner: Raduazo; Henry E.
Attorney, Agent or Firm: Foley & Lardner
Parent Case Text
This application is a continuation, of application Ser. No. 08/145,305,
filed Nov. 3, 1993 now abandoned, which in turn is a continuation of application
Ser. No. 07/622,968, filed Dec. 6, 1990 and now abandoned.
Claims
What is claimed is:
1. A hydroponic nutrient solution control
system for controlling nutrient solution to be supplied to plants to be grown,
comprising:
nutrient solution tank means for containing the nutrient
solution to be supplied to the plants, to which the nutrient solution supplied
to the plants are returned;
pH meter means for measuring pH of the
nutrient solution in the nutrient solution tank means;
an ion analyzer
device for measuring an ion concentration of a plurality of nutrient ion
ingredients of the nutrient solution in the nutrient solution tank means
independent of other nutrient ion ingredients of the nutrient solution in the
nutrient solution tank means;
a plurality of acid solution tank means
for containing acid nutrient ion ingredient solutions, each acid solution tank
means separately containing an acid nutrient ion ingredient solution comprising
a compound selected from the group consisting of HNO.sub.3, H.sub.3 PO.sub.4,
and H.sub.2 SO.sub.4, to be supplied to the nutrient solution in the nutrient
solution tank means;
a plurality of alkali solution tank means for
containing alkali nutrient ion ingredient solutions, each alkali solution tank
means separately containing an alkali nutrient ion ingredient solution
comprising a compound selected from the group consisting of KOH, Ca(OH).sub.2,
Mg(OH).sub.2, and NH.sub.4 OH, to be supplied to the nutrient solution in the
nutrient solution tank means; and
means for automatically controlling
supplies of the acid nutrient ion ingredient solutions and the alkali nutrient
ion ingredient solutions by selectively operating appropriate ones of the acid
solution tank means and alkali solution tank means, according to the pH measured
by the pH meter means and the ion concentrations measured by the ion analyzer
device.
2. The system of claim 1, wherein the controlling means controls
the supplies of the acid nutrient ion ingredient solutions and the alkali
nutrient ion ingredient solutions from the acid solution tank means and the
alkali solution tank means, such that when the pH measured by the pH meter means
is lower than a prescribed acceptable range, those alkali nutrient ion
ingredient solutions whose ion concentrations are measured to be relatively low
by the ion analyzer means are supplied from the alkali solution tank means,
whereas when the pH measured by the pH meter means is higher than a prescribed
acceptable range, those acid nutrient ion ingredient solutions whose ion
concentrations are measured to be relatively low by the ion analyzer means are
supplied from the acid solution tank means.
3. The system of claim 2,
wherein the controlling means also controls the supplies of the acid nutrient
ion ingredient solutions and the alkali nutrient ion ingredient solutions from
the acid solution tank means and the alkali solution tank means, when at least
one of the ion concentrations measured by the ion analyzer means is outside a
prescribed acceptable range, in order to bring said at least one of the ion
concentrations to the prescribed acceptable range.
4. The system of
claim 3, wherein the controlling means controls the supplies of the acid
nutrient ion ingredient solutions and the alkali nutrient ion ingredient
solutions from the acid solution tank means and the alkali solution tank means,
such that when at least one of the ion concentrations for positive ions measured
by the ion analyzer means is lower than the prescribed acceptable range, those
alkali nutrient ion ingredient solutions which contain said positive ions are
supplied from the alkali solution tank means, whereas when at least one of the
ion concentrations for negative ions measured by the ion analyzer means is lower
than the prescribed acceptable range, those acid nutrient ion ingredient
solutions which contain said negative ions are supplied from the acid solution
tank means.
5. The system of claim 3, wherein the controlling means
controls the pH of the nutrient solution in the nutrient solution tank means
again after the supplies of the acid nutrient ion ingredient solutions and the
alkali nutrient ion ingredient solutions from the acid solution tank means and
the alkali solution tank means are controlled in order to bring said at least
one of the ion concentrations to the prescribed acceptable range.
6. The
system of claim 1, further comprising:
electrolytic conductivity meter
means for measuring electrolytic conductivity of the nutrient solution in the
nutrient solution tank means;
high concentration nutrient solution tank
means for containing high concentration nutrient solutions to be supplied to the
nutrient solution in the nutrient solution tank means; and
water supply
means for supplying water to be supplied to the nutrient solution in the
nutrient solution tank means;
and wherein the controlling means also
controls the supplies of the high concentration nutrient solutions and the water
from the high concentration nutrient solution tank means and the water supply
means according to the electrolytic conductivity measured by the electrolytic
conductivity meter means.
7. The system of claim 6, wherein the
controlling means also controls the supplies of the acid nutrient ion ingredient
solutions, the alkali nutrient ion ingredient solutions, and the water from the
acid solution tank means, the alkali solution tank means, and the water supply
means, such that when the ion concentrations for at least one nutrient ion
ingredient measured by the ion analyzer means is higher than the prescribed
acceptable range, those alkali nutrient ion ingredient solutions and those acid
nutrient ion ingredient solutions which do not contain said at least one
nutrient ion ingredient are supplied from the alkali solution tank means and the
acid solution tank means until the ion concentrations for all the nutrient ion
ingredients are balanced, and then the water is supplied from the water supply
means until the electrolytic conductivity of the nutrient solution in the
nutrient solution tank means is restored to that before the supplies of the acid
nutrient ion ingredient solutions and the alkali nutrient ion ingredient
solutions from the acid solution tank means and the alkali solution tank means.
8. The system of claim 7, wherein the controlling means controls the pH
of the nutrient solution in the nutrient solution tank means again after the
supplies of the acid nutrient ion ingredient solutions, the alkali nutrient ion
ingredient solutions, and the water from the acid solution tank means, the
alkali solution tank means, and the water supply means are controlled in order
to bring said at least one of the ion concentrations to the prescribed
acceptable range.
9. A hydroponic nutrient solution control system for
continuous controlling the individual nutrient ion content of a nutrient
solution to be supplied to plants to be grown, comprising:
nutrient
solution tank means for containing the nutrient solution to be supplied to the
plants, to which the nutrient solution supplied to the plants are returned;
pH meter means for measuring pH of the nutrient solution in the nutrient
solution tank means;
an ion analyzer device for measuring an ion
concentration of a plurality of nutrient ion ingredients of the nutrient
solution in the nutrient solution tank means independent of other nutrient ion
ingredients of the nutrient solution in the nutrient solution tank means;
a plurality of acid solution tank means for containing acid nutrient ion
ingredient solutions, each acid solution tank means separately containing an
acid nutrient ion ingredient solution comprising a compound selected from the
group consisting of HNO.sub.3, H.sub.3 PO.sub.4, and H.sub.2 SO.sub.4, to be
supplied to the nutrient solution in the nutrient solution tank means, wherein
each acid nutrient ion ingredient solution is capable of both lowering the
effective pH of the nutrient solution and raising the concentration of a single
nutrient ion contained in the nutrient solution;
a plurality of alkali
solution tank means for containing alkali nutrient ion ingredient solutions,
each alkali solution tank means separately containing an alkali nutrient ion
ingredient solution comprising a compound selected from the group consisting of
KOH, Ca(OH).sub.2, Mg(OH).sub.2, and NH.sub.4 OH, to be supplied to the nutrient
solution in the nutrient solution tank means, wherein each alkali nutrient ion
ingredient solution is capable of both raising the effective pH of the nutrient
solution and raising the concentration of a single specific nutrient ion
contained in the nutrient solution; and
means for automatically
controlling supplies of the acid nutrient ion ingredient solutions and the
alkali nutrient ion ingredient solutions by selectively operating appropriate
ones of the acid solution tank means and alkali solution tank means, according
to the pH measured by the pH meter means and the ion concentrations measured by
the ion analyzer device.
Description
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to a hydroponic nutrient solution control
system for growing plants hydroponically by controlling supplies of nutrient
solutions (solutions of inorganic fertilizers in water) to the plants.
2. Description of the Background Art
In the hydroponics, the
plants are grown under the control of a hydroponic nutrient solution control
system which controls supplies of nutrient solutions to the plants.
In a
conventional hydroponic nutrient solution control system, this controlling is
performed according to the pH and/or the electrolytic conductivity of the
nutrient solutions.
For instance, when the electrolytic conductivity of
the nutrient solution decreased, high concentration nutrient solutions are
supplied to the nutrient solution in order to raise the electrolytic
conductivity to prescribed target range, whereas when the electrolytic
conductivity of the nutrient solution increased, water is added to the nutrient
solution in order to lower the electrolytic conductivity to prescribed target
range.
Similarly, in a case of using the pH, an acid solution and an
alkali solution are used to control the pH of the nutrient solution within a
prescribed target range.
However, in such a conventional hydroponic
nutrient solution control system, it has been difficult to accurately supply the
nutrient solutions containing the ingredients necessary for the plants to be
grown. This is due to the fact that in a course of the growth of the plants, the
organic acids are leaked from the roots of the plants, such that even when the
ingredients unnecessary for the plants are being compiled, this fact is not
reflected by the pH or the electrolytic conductivity of the nutrient solutions.
As a consequence, the necessary adjustments of the nutrient solutions cannot be
provided, so that the growth of the plants are retarded if not hampered fatally.
Also, because the information on the pH and the electrolytic
conductivity cannot reflect the actual state of the nutrient solutions
accurately, even when the abnormality was found in the growing plants, the cause
of the abnormality cannot be ascertained from the information on the pH and the
electrolytic conductivity, so that the entire nutrient solutions have to be
replaced in such a case, which increases the waste of the nutrient solutions.
Also, in a conventional hydroponic nutrient solution control system, the
controlling of the pH is performed by using one acid solution and one alkali
solution only. However, necessary amounts of the acid solution or the alkali
solution vary from time to time, so that it is desirable to have more than one
acid solutions and more than one alkali solutions, in order to be able to cope
with various different situations.
It is known that a plant in a process
of growing may consume particular ingredients more than other ingredients,
depending on the external conditions such as an amount of sunshine, a
temperature of the air, and a temperature of the nutrient solution. In such a
case, the nutrient solution control according to the pH or the electrolytic
conductivity tends to cause a lack of particular nutrient solution ion
ingredients. In this state, the plant is unable to absorb the necessary ion
ingredients because of their low concentration in the nutrient solution.
On the other hand, the ion ingredients in the nutrient solution cannot
be controlled easily, because the ions exist in forms of bases such as
KNO.sub.3.
Furthermore, when the concentration of a particular kind of
ions is very low, the balance of ions achieved in the original nutrient solution
is often lost, such that the pH of the nutrient solution often differs greatly
from the original value.
SUMMARY OF THE INVENTION
It is
therefore an object of the present invention to provide a hydroponic nutrient
solution control system capable of performing the nutrient solution control
automatically, accurately, effectively, and efficiently.
According to
one aspect of the present invention there is provided a hydroponic nutrient
solution control system for controlling nutrient solution to be supplied to
plants to be grown, comprising: nutrient solution tank means for containing the
nutrient solution to be supplied to the plants, to which the nutrient solution
supplied to the plants are returned; pH meter means for measuring pH of the
nutrient solution in the nutrient solution tank means; ion analyzer means for
measuring ion concentrations of nutrient ion ingredients of the nutrient
solution in the nutrient solution tank means; acid solution tank means for
containing acid nutrient ion ingredient solutions to be supplied to the nutrient
solution in the nutrient solution tank means; alkali solution tank means for
containing alkali nutrient ion ingredient solutions to be supplied to the
nutrient solution in the nutrient solution tank means; and means for controlling
supplies of the acid nutrient ion ingredient solutions and the alkali nutrient
ion ingredient solutions from the acid solution tank means and the alkali
solution tank means, according to the pH measured by the pH meter means and the
ion concentrations measured by the ion analyzer means.
According to
another aspect of the present invention there is provided a method of hydroponic
nutrient solution control for controlling nutrient solution to be supplied to
plants to be grown, comprising the steps of: providing nutrient solution tank
means for containing the nutrient solution to be supplied to the plants, to
which the nutrient solution supplied to the plants are returned; measuring pH of
the nutrient solution in the nutrient solution tank means by pH meter means;
measuring ion concentrations of nutrient ion ingredients of the nutrient
solution in the nutrient solution tank means by ion analyzer means; providing
acid solution tank means for containing acid nutrient ion ingredient solutions
to be supplied to the nutrient solution in the nutrient solution tank means;
providing alkali solution tank means for containing alkali nutrient ion
ingredient solutions to be supplied to the nutrient solution in the nutrient
solution tank means; and controlling supplies of the acid nutrient ion
ingredient solutions and the alkali nutrient ion ingredient solutions from the
acid solution tank means and the alkali solution tank means, according to the pH
measured by the pH meter means and the ion concentrations measured by the ion
analyzer means.
Other features and advantages of the present invention
will become apparent from the following description taken in conjunction with
the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
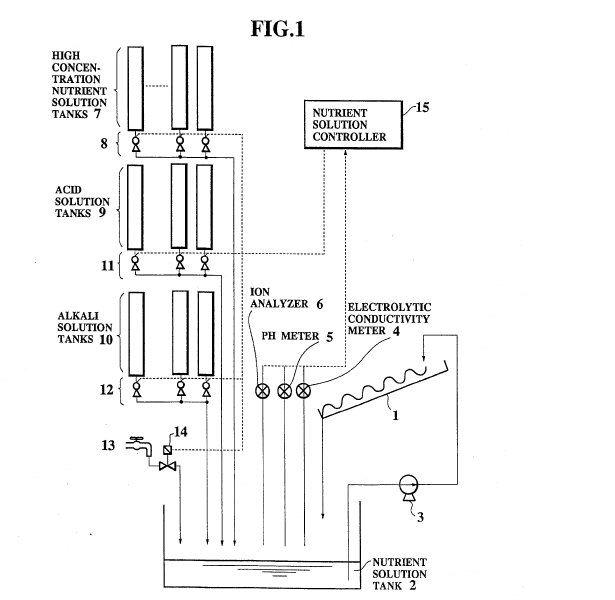
FIG. 1 is a block diagram of one embodiment of a hydroponic nutrient
solution control system according to the present invention.
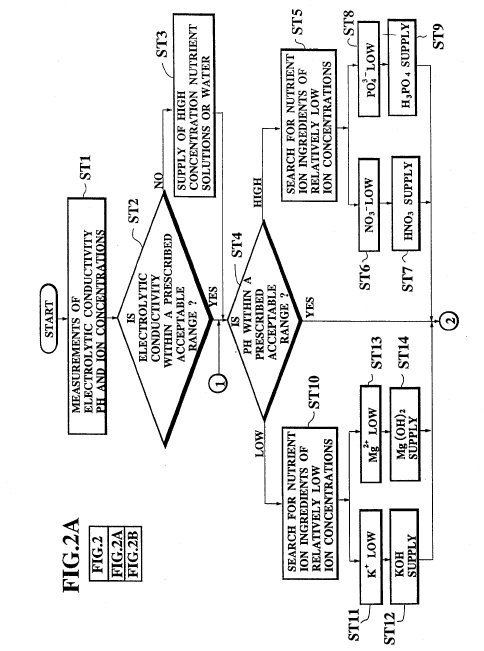
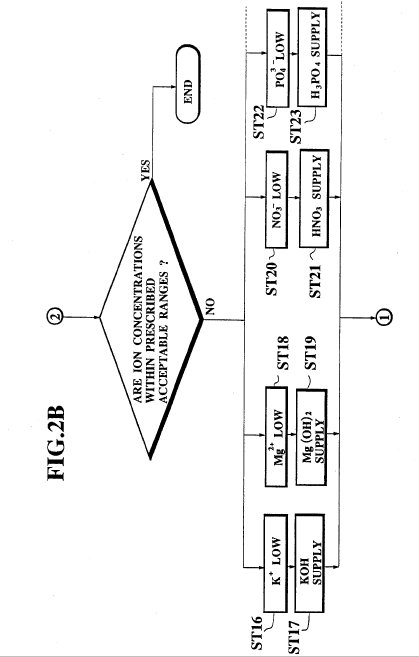
FIG. 2 which
has been divided into two parts (FIG. 2A and FIG. 2B) is a flow chart for the
nutrient solution control operation performed by the system of FIG. 1.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Referring now
to FIG. 1, one embodiment of a hydroponic nutrient solution control system
according to the present invention will be described in detail.
This
system is adapted to a so called NFT (nutrient flow technique) culture method,
and comprises a culture panel 1 on which plants to be grown are planted, a
nutrient solution tank 2 for containing the nutrient solution to be supplied to
the plants, and a supply pump 3 for supplying the nutrient solution in the
nutrient solution tank 2 to an upper stream side of the culture panel 1 from
which the nutrient solution flows down through a lower stream side of the
culture panel 1 back to the nutrient solution tank 2 while supplying the
nutrient ingredients to the plants.
The system further comprises an
electrolytic conductivity meter 4 for measuring an electrolytic conductivity of
the nutrient solution in the nutrient solution tank 2 which is proportional to a
concentration of the nutrient solution and therefore is indicative of the total
ion concentration of the nutrient solution as a whole, a pH meter 5 for
measuring a balance of acid and alkali, i.e., pH of the nutrient solution in the
nutrient solution tank 2, and an ion analyzer 6 for analyzing ion contents and
measuring ion concentrations of the nutrient solution in the nutrient solution
tank 2. The multi-ion meter LQ201 of Toshiba which uses the ion electrode method
is suitable for the ion analyzer 6 of this system.
The system further
comprises a plurality of high concentration nutrient solution tanks 7 for
containing several high concentration nutrient solutions to be supplied to the
nutrient solution tank 2, a plurality of high concentration nutrient solution
tank pumps 8 capable of controlling amounts of the high concentration nutrient
solutions flowing out from the high concentration nutrient solution tanks 7, a
plurality of acid solution tanks 9 and a plurality of alkali solution tanks 10
for containing several acid ingredient solutions and several alkali ingredient
solutions, respectively, to be supplied to the nutrient solution tank 2, a
plurality of acid solution tank pumps 11 and a plurality of alkali solution tank
pumps 12 capable of controlling amounts of the acid ingredient solutions and the
alkali ingredient solutions flowing out from the acid solution tank 9 and the
alkali solution tank 10, respectively, a water supply 13 for supplying water to
dilute the nutrient solution in the nutrient solution tank 2, a water supply
electromagnetic valve 13 capable of controlling an amount of water flowing out
from the water supply 13, and a nutrient solution controller 15 for controlling
the high concentration nutrient solution tank pumps 8, acid solution tank pumps
11, alkali solution tank pumps 12, and a water supply electromagnetic valve 14,
according to the electrolytic conductivity, pH, and ion concentrations of ion
contents obtained by the electrolytic conductivity meter 4, pH meter 5, and ion
analyzer 6.
The high concentration nutrient solution tanks 7 separately
contain various high concentration nutrient solutions which includes various
nutrient ingredient ions at high concentrations.
The acid solution tanks
9 separately contain various acid nutrient ion ingredient solutions including
those of the major nutrient ion ingredients such as HNO.sub.3, H.sub.3 PO.sub.4,
and H.sub.2 SO.sub.4, as well as those of minor nutrient ion ingredients such as
H.sub.3 BO.sub.4 and H.sub.2 MoO.sub.4.
The alkali solution tanks 10
separately contain various alkali nutrient ion ingredient solutions including
those of the major nutrient ion ingredients such as KOH, Ca(OH).sub.2,
Mg(OH).sub.2 and NH.sub.4 OH, as well as those of minor nutrient ion ingredients
such as Fe(OH).sub.2, Fe(OH).sub.3, Mn(OH).sub.2, Zn(OH).sub.2, and
Cu(OH).sub.2.
This system operates under the control of the nutrient
solution controller 15 according to the flow chart of FIG. 2, as follows.
Initially, in preparing new nutrient solution, the high concentration
nutrient solution tank pumps 8 and the water supply electromagnetic valve 14 are
controlled according to the growth level of the plants to be grown, so as to
fill the nutrient solution tank 2 with the appropriate nutrient solution
containing all the nutrient ion ingredients necessary for the plants mixed
evenly.
As the supply pump 3 supplies this nutrient solution in the
nutrient solution tank 2 to the upper stream side of the culture panel 1 from
which the nutrient solution flows down through the lower stream side of the
culture panel 1 back to the nutrient solution tank 2 while supplying the
nutrient ion ingredients to the plants, those nutrient ion ingredients which are
easier to be absorbed decrease, and the pH of the nutrient solution changes.
In order to control such a situation, first at the step ST1, the
nutrient solution controller 15 collects the electrolytic conductivity, pH, and
ion concentrations of ion contents of the nutrient solution in the nutrient
solution tank 2 measured by the electrolytic conductivity meter 4, pH meter 5,
and ion analyzer 6 through regular constant interval samplings.
Next, at
the step ST2, whether the measured electrolytic conductivity is within a
prescribed acceptable range or not is determined. When the measured electrolytic
conductivity is found to be within the prescribed acceptable range, the step ST4
will be taken next. Otherwise, next at the step ST3, when the measured
electrolytic conductivity is found to be too low, the high concentration
nutrient solution tank pumps 8 are controlled such that appropriate amounts of
the high concentration nutrient solutions to raise the electrolytic conductivity
of the nutrient solution up to the prescribed acceptable range are supplied to
the nutrient solution tank 2, whereas when the measured electrolytic
conductivity is found to be too high, the water supply electromagnetic valve 14
is controlled such that appropriate amount of the water to lower the
electrolytic conductivity of the nutrient solution up to the prescribed
acceptable range is supplied to the nutrient solution tank 2.
Then at
the step ST4, whether the measured pH is within a prescribed acceptable range or
not is determined. When the measured pH is found to be within the prescribed
acceptable range, the step ST15 will be taken next.
Otherwise, when the
measured pH is found to be too high, i.e., when the nutrient solution is
excessively alkalic, those nutrient ion ingredients whose ion concentrations are
found to be relatively low are supplied from the acid solution tanks 9 in forms
of acid ingredient solutions by controlling the acid solution tank pumps 11, so
as to bring the pH of the nutrient solution to the prescribed acceptable range.
Namely, in this case, next at the step ST5, the ion concentrations of the ion
contents of the nutrient solution are checked to find those nutrient ion
ingredients whose ion concentrations are found to be relatively low. Then, when
the ion concentration for NO.sub.3 - is found to be relatively low (a case of
step ST6) for example, an appropriate amount of the HNO.sub.3 solution is
supplied at the step ST7, whereas when the ion concentration for PO.sub.4.sup.3-
is found to be relatively low (a case of step ST8) for example, an appropriate
amount of the H.sub.3 PO.sub.4 solution is supplied at the step ST9.
On
the other hand, when the measured pH is found to be too low, i.e., when the
nutrient solution is excessively acidic, those nutrient ion ingredients whose
ion concentrations are found to be relatively low are supplied from the alkali
solution tanks 10 in forms of alkali ingredient solutions by controlling the
alkali solution tank pumps 12, so as to bring the pH of the nutrient solution to
the prescribed acceptable range. Namely, in this case, next at the step ST10,
the ion concentrations of the ion contents of the nutrient solution are checked
to find those nutrient ion ingredients whose ion concentrations are found to be
relatively low. Then, when the ion concentration for K.sup.+ is found to be
relatively low (a case of step ST11) for example, an appropriate amount of the
KOH solution is supplied at the step ST12, whereas when the ion concentration
for Mg.sup.2+ is found to be relatively low (a case of step ST13) for example,
an appropriate amount of the Mg(OH).sub.2 solution is supplied at the step ST14.
Then, at the step ST15, the ion concentrations of the ion contents of
the nutrient solution are checked to find those nutrient ion ingredients whose
ion concentrations are found to be lower than prescribed acceptable ranges. This
step is taken because the ion concentrations for all the nutrient ion
ingredients of the nutrient solution may not necessarily be within the
prescribed acceptable ranges, even when the pH of the nutrient solution is
within the prescribed acceptable range. Thus, when the ion concentrations for
all the nutrient ion ingredients of the nutrient solution are found to be within
prescribed acceptable ranges, the controlling process by the nutrient solution
controller 15 is terminated until the next samplings of the electrolytic
conductivity, pH and ion concentrations.
Otherwise, when the ion
concentration for positive ions such as K.sup.+ or Mg.sup.2+ is found to be
lower than the prescribed acceptable range (a case of step ST16 or ST18) for
example, an appropriate amount of the KOH solution or Mg(OH).sub.2 solution is
supplied at the step ST17 or ST19, respectively, whereas when the ion
concentration for negative ions such as NO.sub.3.sup.- or PO.sub.4.sup.3- is
found to be lower than the prescribed acceptable range (a case of step ST20 or
ST22) for example, an appropriate amount of the HNO.sub.3 solution or H.sub.3
PO.sub.4 solution is supplied at the step ST21 or ST23, respectively, so as to
bring ion concentrations for all the nutrient ion ingredients of the nutrient
solution to the prescribed acceptable ranges.
In a case the ion
concentrations for certain nutrient ion ingredients are found to be higher than
the prescribed acceptable ranges at the step ST15, the supply from the acid
solution tanks 9 or the alkali solution tanks 10 which contain solutions of such
nutrient ion ingredients are stopped while the supply from all the other acid
solution tanks 9 and the alkali solution tanks 10 are continued so as to achieve
the overall balance for the ion concentrations for all the nutrient ion
ingredients of the nutrient solution, and then the entire nutrient solution is
diluted by the water from the water supply 13 in order to restore the original
overall ion concentration of the nutrient solution as a whole.
Then, the
process returns to the step ST4 described above, so as to check the pH of the
nutrient solution once again, because the pit may be changed as a result of the
ion concentration control at the steps ST16 to ST23.
Thus, according to
this system, not only the electrolytic conductivity and pH, but also the ion
concentrations for the nutrient ion ingredients of the nutrient solution are
controlled, so that the nutrient solution control can be performed more
accurately and effectively.
Also, according to this system, the pH of
the nutrient solution is controlled according to the ion concentrations for the
nutrient ion ingredients of the nutrient solution, so that the overuse and waste
of the acid or alkali solutions for the purpose of pH controlling can be
prevented, and therefore the nutrient solution control can be performed more
efficiently and stably.
Furthermore, the entire nutrient solution
control can be performed under the controlling by the nutrient solution
controller 15 which can be adapted to different types of plants to be grown,
different conditions in which the plants are to be grown, or different growth
levels of the plants to be grown, by the appropriate programming according to
the most suitable nutrient solution for a given situation, so that the entire
nutrient solution control can be performed automatically.
It is to be
noted that, although the above embodiment has been described for the system
adapted to the NFT culture method, it should be obvious that the present
invention is equally applicable to the system for the other culture method such
as a rock wool culture method and a DFT (deep flow technique) culture method.
Besides this, many modifications and variations of the above embodiments
may be made without departing from the novel and advantageous features of the
present invention. Accordingly, all such modifications and variations are
intended to be included within the scope of the appended claims.
* * * * *
![[US Patent & Trademark Office, Patent Full Text and Image Database]](United States Patent 5,598,663.files/patfthdr.gif)
![[Home]](United States Patent 5,598,663.files/home.gif)
![[Boolean Search]](United States Patent 5,598,663.files/boolean.gif)
![[Manual Search]](United States Patent 5,598,663.files/manual.gif)
![[Number Search]](United States Patent 5,598,663.files/number.gif)
![[Help]](United States Patent 5,598,663.files/help.gif)
![[HIT_LIST]](United States Patent 5,598,663.files/hitlist.gif)
![[PREV_DOC]](United States Patent 5,598,663.files/prevdoc.gif)
![[NEXT_DOC]](United States Patent 5,598,663.files/nextdoc.gif)
![[Bottom]](United States Patent 5,598,663.files/bottom.gif)
![[View Shopping Cart]](United States Patent 5,598,663.files/cart.gif)
![[Add to Shopping Cart]](United States Patent 5,598,663.files/order.gif)
![[Image]](United States Patent 5,598,663.files/image.gif)
![[US Patent & Trademark Office, Patent Full Text and Image Database]](United States Patent 5,598,663.files/patfthdr.gif)
![[Home]](United States Patent 5,598,663.files/home.gif)
![[Boolean Search]](United States Patent 5,598,663.files/boolean.gif)
![[Manual Search]](United States Patent 5,598,663.files/manual.gif)
![[Number Search]](United States Patent 5,598,663.files/number.gif)
![[Help]](United States Patent 5,598,663.files/help.gif)
![[HIT_LIST]](United States Patent 5,598,663.files/hitlist.gif)
![[PREV_DOC]](United States Patent 5,598,663.files/prevdoc.gif)
![[NEXT_DOC]](United States Patent 5,598,663.files/nextdoc.gif)
![[Bottom]](United States Patent 5,598,663.files/bottom.gif)
![[View Shopping Cart]](United States Patent 5,598,663.files/cart.gif)
![[Add to Shopping Cart]](United States Patent 5,598,663.files/order.gif)
![[Image]](United States Patent 5,598,663.files/image.gif)


