![[US Patent & Trademark Office, Patent Full Text and Image Database]](United States Patent 5,858,202.files/patfthdr.gif)
| United States Patent |
5,858,202 |
| Nakamura |
January 12, 1999 |
Method for producing electrolytic water and apparatus for
producing the same
Abstract
The invention provides a method and apparatus for producing an electrolytic
water whereby one electrolytic water used in compliance with its intended use is
transformed into a water more effectively used and the by-product electrolytic
water, previously disposed as virtually useless, is transformed into a water
which can be effectively used by use of at least two electrolyzer in series. At
least one of an outlet for a primary anodic electrolytic water in a primary
electrolyzer and an outlet for a primary cathodic electrolytic water in a
primary electrolyzer is connected to an inlet of a secondary electrolyzer
through a first switching valve and a second switching valve. The anodic
electrolytic water only, the primary cathodic electrolytic water only, or
mixture of the primary anodic and primary cathodic electrolytic water is fed to
the secondary electrolyzer to be electrolyzed again. A chlorine remover is
provided within the secondary inlet for connecting the primary electrolyzer with
the secondary electrolyzer and it removes chlorine from a primary electrolytic
water, and afterward, the filtered water is electrolyzed again in the secondary
electrolyzer. Tertiary electrolysis of the secondary anodic electrolytic water,
produced by electrolysis of the primary anodic electrolytic water, will produce
a tertiary anodic electrolytic water that exhibits a remarkably high
bactericidal effect.
| Inventors: |
Nakamura; Tadamasa (Tokyo, JP) |
| Assignee: |
Zenkoku-Mokko-Kikai-Kan, Inc. (JP)
|
| Appl. No.: |
788196 |
| Filed: |
January 24, 1997 |
Foreign Application Priority Data
|
Jan 30, 1996[JP] |
8-014534 |
| Current U.S. Class: |
205/746; 204/257; 204/278.5
|
| Intern'l Class: |
C02F 001/461 |
| Field of Search: |
205/746,770 204/257,275
|
References Cited [Referenced
By]
U.S. Patent Documents
Primary
Examiner: Phasge; Arun S.
Attorney, Agent or Firm: Lorusso &
Loud
Claims
What is claimed is:
1. An apparatus for producing an
electrolytic water, said apparatus comprising:
a primary electrolyzer
including a primary anode and a primary cathode and a separating membrane
dividing the interior of the primary electrolyzer into a primary anode
compartment containing the primary anode and a cathode compartment containing
the primary cathode;
a secondary electrolyzer including a secondary
anode and a secondary cathode and a separating membrane dividing the secondary
electrolyzer into a secondary anode compartment containing the secondary anode
and a secondary cathode compartment containing the secondary cathode;
a
first primary outlet for primary anodic water connected to the primary anode
compartment;
a first switching valve connected to the first primary
outlet;
a second primary outlet for primary cathodic water connected to
the primary cathode compartment;
a second switching valve connected to
the second primary outlet;
a combined primary outlet passage for
connecting the first switching valve with the second switching valve;
a
secondary inlet connecting the combined primary outlet with the secondary
electrolyzer;
a first secondary outlet for secondary anodic water
connected to the secondary anode compartment;
a second secondary outlet
for secondary cathodic water connected to the secondary cathode compartment; and
a chlorine remover provided in the secondary inlet, between the primary
electrolyzer and the secondary electrolyzer.
2. An apparatus for
producing an electrolytic water as claimed in claim 1, additionally comprising:
a third switching valve upstream of the chlorine remover in the
secondary inlet; and
a bypass connecting the third switching valve with
the secondary inlet at a point downstream of the chlorine remover, whereby water
may be passed either through the chlorine remover or through the bypass by
switching the third switching valve.
3. An apparatus for producing an
electrolytic water as claimed in claim 1, further comprising:
a
secondary anodic water switching valve in the first secondary outlet; and
an anodic water communication passage connecting the secondary anodic
water switching valve with the first switching valve;
whereby the
primary anodic water passing through the first switching valve from the first
primary outlet and the secondary anodic water passing through the secondary
anodic water switching valve from the first secondary outlet may be mixed in the
anodic water communication passage and removed together through the first
primary outlet.
4. An apparatus for producing an electrolytic water as
claimed in claim 1, further comprising:
a secondary cathodic water
switching valve in the second secondary outlet; and
a cathodic water
communication passage connecting the secondary cathodic water switching valve
with the second switching valve;
whereby cathodic water passing through
the second switching valve from the second primary outlet and the cathodic water
passing through the secondary cathodic water switching valve from the second
secondary outlet are mixed in the cathodic water communication passage and
removed together through the second primary outlet.
5. A method for
producing electrolytic water product comprising:
feeding a raw water to
a primary electrolyzer having an interior divided into a primary anode
compartment containing an anode and a primary cathode compartment containing a
cathode;
electrolyzing the raw water within the primary electrolyzer to
produce a primary anodic water and a primary cathodic water;
removing
chlorine by passing at least one said primary anodic water and said primary
cathodic water through a chlorine filter to produce a chlorine free water;
feeding the chlorine free water to a secondary electrolyzer having an
interior divided into a secondary anode compartment containing an anode and a
secondary cathode compartment containing a cathode; and
electrolyzing
the chlorine free water within the secondary electrolyzer to produce a secondary
anodic water and a secondary cathodic water.
6. A method for producing
an electrolytic water as claimed in claim 5, wherein the primary anodic water
only is passed through the chlorine filter and electrolyzed in the secondary
electrolyzer.
7. A method for producing an electrolytic water as claimed
in claim 5, further comprising:
feeding at least one of the secondary
anodic water and the secondary cathodic water to a tertiary electrolyzer having
an interior divided into a tertiary anode compartment containing an anode and a
tertiary cathode compartment containing a cathode; and
electrolyzing the
feed to the tertiary electrolyzer to produce a tertiary anodic water and a
tertiary cathodic water.
8. A method for producing an electrolytic water
as claimed in claim 5 wherein the primary cathodic water only is passed through
the chlorine filter and electrolyzed in the secondary electrolyzer.
9. A
method for producing an electrolytic water as claimed in claim 5 wherein a
mixture of the primary anodic water and the primary cathodic water is passed
through the chlorine filter and then electrolyzed in the secondary electrolyzer.
Description
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to a method for producing an electrolytic
water whereby the effectiveness of one conventional type of electrolytic water
is enhanced and the other type of electrolytic water, conventionally disposed of
as virtually useless, is transformed into an electrolytic water which can be
effectively used, and to an apparatus for producing the same.
2.
Description of the Related Art
An electrolytic water producer has been
known which produces an electrolytic acid water and electrolytic alkaline water
by electrolyzing water. The main components of the electrolytic water producer
are an electrolyzer and a power supply. The inside of the electrolyzer is
divided into two areas by a separating membrane, in one area of which a positive
electrode is disposed, and in the other area of which a negative electrode is
disposed. Applying a current across both of the electrodes in the electrolyzer
filled with water produces the electrolytic acid water in the area where the
positive electrode is disposed and the electrolytic alkaline water in the area
where the negative electrode is disposed.
The electrolytic alkaline
water is recognized to be effective for depressing an abnormal intestinal
fermentation and is used for a drinking water. The electrolytic acid water is
acknowledged to be effective in bactericidal and astringent actions, and is used
for cleaning and medical treatment. Thus, each type of electrolytic water has
widely been used for enhancing health.
In evaluation of the electrolytic
water, the pH value representing hydrogen ion concentration and the residual
chlorine concentration have been conventionally used. However, as use of
electrolytic water producers has become widespread, even without any significant
differences in the pH value or in the residual chlorine concentration of the
electrolytic water, differences in electrolytic water effectiveness are noted as
dependent on apparatus and region of use.
In the electrolytic alkaline
water, when the oxidation reduction potential and dissolved oxygen concentration
are low, the water usually displays a high effectiveness for improving health;
whereas when the oxidation reduction potential and dissolved oxygen
concentration are comparably high, the water shows a low effectiveness for
improving health in most cases. A low oxidation reduction potential was
considered -50-250 mv, a low dissolved oxygen concentration was considered
4.8-6.8 mg/l, a high oxidation reduction potential was considered +100-+250 mv
and a high dissolved oxygen concentration was considered 7-8.2 mg/l.
Accordingly, using an identical service water as the raw water,
selecting a model A to produce an electrolytic water with a low oxidation
reduction potential and a given pH value, and a model B to produce an
electrolytic water with a comparably higher oxidation reduction potential and an
identical pH value, a comparison test using rats was made simultaneously with
the service water, the electrolytic water produced by the model A, and the
electrolytic water produced by the model B by a single testing organization. The
test results showed an influence on the gastric mucosal damage, i.e. that the
area of erosion on a gastric mucosa tends to become smaller in the following
order: electrolytic water produced by the model A, electrolytic water produced
by the model B, and service water. A survival test using cancer transplanted
immunodefficient mice was made by another testing organization in a similar
manner, and the test confirmed a similar tendency in the survival rate.
The oxidation reduction potential and dissolved oxygen concentration of
the waters used in these tests were on average: +230 mv, 8.2 mg/l for the
service water; -150 mv, 6.2 mg/l for the electrolytic alkaline water produced by
the model A; and +75 mv, 7.6 mg/l for the electrolytic alkaline water produced
by the model B.
Another test on the survival rate using critical
immunodefficient mice made by another organization employed: the service water
with a pH of 7.6, an oxidation reduction potential of +520 mv, and a dissolved
oxygen concentration of 78 mg/l; a first electrolytic alkaline water C
(hereinafter "water C") with a pH of 10.4, an oxidation reduction potential of
-485 mv, and a dissolved oxygen concentration of 5.8 mg/l; and a second
electrolytic alkaline water D (hereinafter "water D") with a pH of 8.9, an
oxidation reduction potential of -309 mv, and a dissolved oxygen concentration
of 7.22 mg/l. The test results reported for the survival rate with the service
water group, water C group, and water D group were 20.9%, 56.0%, 44.0%,
respectively.
According to the aforementioned test results, in the
electrolytic alkaline water, as the oxidation reduction potential and dissolved
oxygen concentration become lower, the effectiveness becomes higher, indicating
need for an apparatus which produces an electrolytic alkaline water with a low
oxidation reduction potential and dissolved oxygen concentration, without being
subject to influence by the state of the raw water.
The electrolytic
acid water, produced as a waste water in producing the electrolytic alkaline
water for drinking, is considered to have an astringent action and a
bactericidal action. However, its effectiveness is not so apparent as to be
really recognized in most cases, and most of such acidic waters are disposed as
waste.
When the pH decreases to about 4, the oxidation reduction
potential becomes more than +800 mv, and the dissolved oxygen concentration
becomes more than 10 mg/l, the electrolytic acid water clearly displays the
astringent action when applied to the skin and makes the skin smooth after the
water dries. The electrolytic acid water gives the smooth feeling to the skin
stronger and longer, as the pH value becomes lower, the oxidation reduction
potential becomes higher, and the dissolved oxygen concentration becomes higher.
The bacteriostatic effect also has a similar tendency. For example, if the pH
value is less than 3.5, the oxidation reduction potential is more than +900 mv,
and the dissolved oxygen concentration is more than 12 mg/l, the electrolytic
acid water will exhibit such a strong bactericidal activity as to kill most
bacteria in a short time even with a dissolved chlorine concentration of about 2
ppm, indicating possible utility as an effective antibacterial agent that does
not damage the skin or mocosa.
However, the conventional apparatus can
not stably produce an effective acid electrolytic water, because the water
quality of the raw water, e.g. service water, changes significantly with the
seasons, with temperature, and with time. Furthermore, the conventional
apparatus required such maintenance control that an electrolyte solution of a
specific concentration always has to be kept so as not to be out of stock.
The raw water fed to the electrolytic water producer sometimes contains
free chlorine as hypochlorite, iron rust, and turbidity. In such a case, it is a
common practice to pass the raw water through a chlorine remover having a filter
for rejecting chlorine of an activated carbon alone or a combination of an
activated carbon, hollow fiber membranes and calcium sulfites, and afterward to
feed the raw water into the electrolytic water producer. Furthermore, where the
major objective is producing an electrolytic water with bactericidal activity,
it is also a common practice to add chlorides such as a sodium chloride and/or
potassium chloride to the raw water as electrolytes.
There are various
types of such apparatus: one type employing a constant current power supply as
the power supply to the electrodes in order to stabilize electrolysis; apparatus
capable of switching the ranges of a current from a constant current power
supply; and apparatus with a pH controller that measures the electric
conductivity of an electrolytic water and feeds back the measured result to the
electrolyzing power supply.
In the case of the apparatus capable of
producing the electrolytic alkaline water for drinking such that the pH value
does not exceed 11, the oxidation reduction potential will vary in the range
from +150 mv to -250 mv and will be unstable, depending on the potential of the
raw water, the gas dissolved in the raw water, the electrolytes contained in the
raw water, and the quantity of water treated.
In case of the apparatus
provided with the pH controller, it is possible to produce an electrolytic water
with a comparably stable pH value; however, the oxidation reduction potential
changes significantly, depending on differences in the raw water as in the
previous case.
When chlorides are added as electrolytes, it is possible
to produce an electrolytic acid water with a low pH value and a high oxidation
reduction potential; however, this technique is prone to a high free dissolved
chlorine concentration of more than 50-150 ppm and it has proven very difficult
to reliably suppress the flee dissolved chlorine concentration to less than a
specific limit.
On the other hand, the electrolytic acid water
containing free chlorine has a bactericidal effect. For instance, in the
treatment of atopic dermatitis with a serious secondary infection, a high
chlorine concentration displays a significant effect of skin disinfection. The
free dissolved chlorine concentration contained in an electrolytic acid water
does not damage a healthy skin up to about 50 ppm; however, if the electrolytic
acid water is repeatedly applied several times a day to a skin that is chapped
or inflamed or anaphylactic, the electrolytic acid water with a chlorine
concentration of more than 25 ppm reportedly causes a slight damage such as
eruption owing to irritation. Therefore, the chlorine concentration should be
controlled to less than 20 ppm for safety, except for use under supervision of a
doctor.
Furthermore, electrolytic alkaline water coproduced with
electrolytic acid water having a strong bactericidal effect will sometimes have
a high pH value exceeding pH 11 and a low oxidation reduction potential of less
than -800 mv. Such an electrolytic alkaline water is rich in metal ions such as
sodium and potassium ions derived from electrolytes, and drinking such an
electrolytic alkaline water is a danger to one's physical condition and it is
very bad tasting. Accordingly, such alkaline water has been disposed as waste,
although it can be used for cleaning utensils and the like.
Generally,
the quantity of added electrolytes is in the range of 500.+-.200 mg/l as sodium
chloride. In the case of chlorine ions as electrolytes contained in the service
water as a raw water, the maximum level of chlorine ion content consistent with
water quality standards is 200 mg/l (329 mg/l as NaCl). Accordingly, in the
electrolyte addition type apparatus also, the quality of the raw water specially
significantly influences the oxidation reduction potential and free dissolved
chlorine concentration of the electrolytic water thereby produced.
In
the conventional apparatus, the electrolysis is conducted in one electrolyzer;
and for a raw water of constant quality, the quantity of water to be treated and
the amount of current to be applied will determine a quantitative combination of
a pH value, oxidation reduction potential, dissolved oxygen concentration, free
dissolved chlorine concentration, electric conductivity, and the like.
Consequently, it was difficult to simultaneously produce an electrolytic water
with a different quantitative combination of these and also difficult to produce
an electrolytic alkaline water suitable for drinking and an electrolytic acid
water with a bactericidal and bacteriostatic effect.
Furthermore, the
conventional apparatus is designed without paying attention to the function of
dissolved oxygen and, in fact, the dissolved oxygen concentration of the
electrolytic water is completely disregarded.
SUMMARY OF THE INVENTION
The present invention has been made in view of the foregoing problems,
and an object of the present invention is to provide a method for producing an
acidic or alkaline type electrolytic water which can be more effectively used by
repeating electrolysis more than two times and the other type electrolytic
water, conventionally disposed as virtually useless, as an electrolytic water
which can also be effectively used, and to provide an apparatus for producing
the same.
Another object of the invention is to provide an apparatus in
which any one of an anodic electrolytic water or cathodic electrolytic water
obtained by primary electrolysis or a mixed water of these can be selected and
fed into a secondary electrolyzer so as to be secondarily electrolyzed.
Another object of the invention is to provide a method for producing an
electrolytic water with a remarkably high bactericidal effect.
In order
to accomplish the aforementioned objects, in a method for producing an
electrolytic water, according to one aspect of the present invention, a water is
electrolyzed into an anodic electrolytic water and a cathodic electrolytic water
in a primary electrolyzer having an area provided with an anode and an area
provided with a cathode divided by a separating membrane, and a water produced
in the primary electrolyzer is secondarily electrolyzed in a secondary
electrolyzer.
Furthermore, according to another aspect of the present
invention, an apparatus is provided for producing an electrolytic water, which
apparatus includes a primary electrolyzer provided with an anode and a cathode
and a separating membrane for dividing the primary electrolyzer into an area
containing the anode and an area containing the cathode, a secondary
electrolyzer provided with an anode and a cathode and a separating membrane for
dividing the secondary electrolyzer into an area containing the anode and an
area containing the cathode. An outlet for primary anodic electrolytic water
connects the area containing the anode of the primary electrolyzer to a first
switching valve, an outlet for a primary cathodic electrolytic water connects to
the area containing the cathode of the primary electrolyzer to a second
switching valve and a combined water passage connects the first switching valve
with the second switching valve. A secondary inlet connects the combined water
passage at one end with the secondary electrolyzer at its other end. Further, an
outlet for a secondary anodic electrolytic water is connected to the area
containing the anode in the secondary electrolyzer, and an outlet for a
secondary cathodic electrolytic water is connected to the area containing the
cathode in the secondary electrolyzer.
According to another aspect of
the invention, in the method for producing an electrolytic water, a primary
anodic electrolytic water is secondarily electrolyzed to produce a secondary
anodic electrolytic water, and the secondary anodic electrolytic water is
electrolyzed to produce a tertiary anodic electrolytic water.
BRIEF
DESCRIPTION OF THE DRAWING
Preferred embodiments of the present
invention will hereinafter be described with reference to the accompanying
drawing.
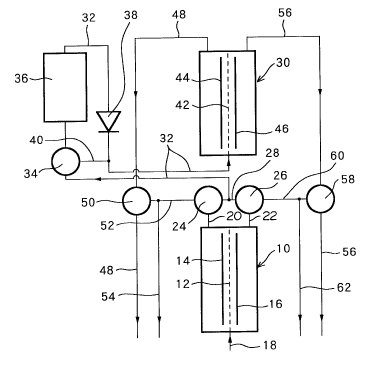
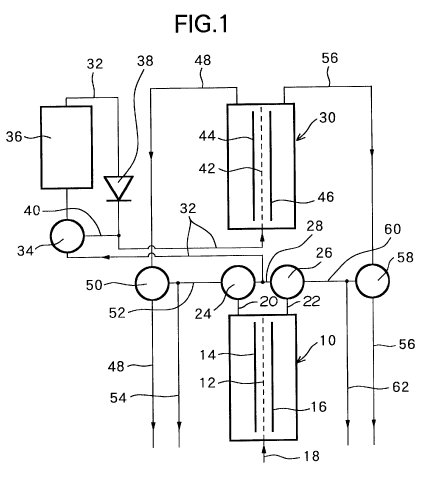
FIG. 1 illustrates an apparatus used for producing an
electrolytic water according to the present invention.
The interior of a
primary electrolyzer 10 is divided into two areas by a separating membrane 12,
in one area of which an positive electrode is provided, and in the other area of
which a negative electrode is provided. The primary electrolyzer 10 is provided
with a primary inlet 18 for receiving a raw water, an outlet 20 for a primary
anodic electrolytic water communicating with the area containing the positive
electrode 14, and an outlet 22 for a primary cathodic electrolytic water
communicating with the area containing the negative electrode 16.
In the
primary electrolyzer 10, the anodic electrolytic water produced at the positive
electrode 14 is removed through outlet 20 as the primary anodic electrolytic
water, i.e., the acid water, and the cathodic electrolytic water produced at the
negative electrode 16 is removed through outlet 22 as the primary cathodic
electrolytic water, i.e., the alkaline water.
The outlet passage 20 for
the primary anodic electrolytic water is connected to a first switching valve
24, the outlet 22 for the primary cathodic electrolytic water is connected to a
second switching valve 26, and a combined outlet 28 connects the first switching
valve 24 with the second switching valve 26.
In addition to the primary
electrolyzer 10, a secondary electrolyzer 30 is provided, and a secondary inlet
passage 32 connects the secondary electrolyzer 30 to the mid-point of the
combined outlet 28. That is, an electrolytic water produced in the primary
electrolyzer 10 is fed into the secondary electrolyzer 30 through the secondary
inlet passage 32. Mid-way of the secondary inlet 32, a third switching valve 34,
chlorine remover 36, and a check-valve 38 are provided in sequence toward the
secondary electrolyzer 30. The chlorine remover 36 has a filter for removing
chlorine using activated carbon or the like. A bypass passage 40 connects the
third switching valve 34 with the secondary incoming passage 32 between the
check-valve 38 and the secondary electrolyzer 30.
The secondary
electrolyzer 30 has the same construction as the primary electrolyzer 10. The
interior of the secondary electrolyzer 30 is divided into two areas by a
separating membrane 42, in one of which areas an anode 44 is provided, and in
the other area a cathode 46 is provided. A secondary anodic electrolytic water
outlet 48 is connected to the area where the anode 44 of the secondary
electrolyzer 30 is disposed, and the anodic electrolytic water electrolyzed in
the secondary electrolyzer 30 is removed through the secondary anodic
electrolytic water outlet 48. A fourth switching valve 50 is provided in the
secondary anodic electrolytic water outlet 48, and an anodic electrolytic water
communicating passage 52 connects the fourth switching valve 50 with the first
switching valve 24. An anodic electrolytic water inlet 54 is connected to the
anodic electrolytic water communicating passage 52.
A secondary cathodic
electrolytic water outlet 56 is connected to the area where the negative
electrode 46 of the secondary electrolyzer 30 is disposed, and the cathodic
electrolytic water electrolyzed in the secondary electrolyzer 30 is taken out
through the secondary cathodic electrolytic water passage 56. A fifth switching
valve 58 is provided on the secondary cathodic electrolytic water outlet 56, and
a cathodic electrolytic water communicating passage 60 connects the fifth
switching valve 58 with the second switching valve 26. A primary cathodic
electrolytic water outlet 62 is connected to the cathodic electrolytic water
communicating passage 60.
An electronic controller (not illustrated)
controls the power supply and voltage regulation for the primary electrolyzer
and the secondary electrolyzer 30 and the switching operations of the switching
valves 24, 26, 34, 50, and 58.
The operation of the apparatus will now
be described, first, for the case wherein only the anodic electrolytic water
produced in the primary electrolyzer 10 is electrolyzed in the secondary
electrolyzer 30. The first switching valve 24 is positioned to connect the
outlet 20 for the primary anodic electrolytic water to the combined outlet 28,
so that the anodic electrolytic water produced in the primary electrolyzer 10 is
fed into the secondary electrolyzer 30 through the combined outlet 28 and the
secondary inlet 32. The third switching valve 34 provided in the secondary inlet
32 is switched so as to connect the combined outlet passage 28 to the bypass 40.
That is, the anodic electrolytic water, produced in the primary electrolyzer 10,
does not pass through the chlorine remover 36.
The second switching
valve 26 is switched to connect the outlet 22 for the primary cathodic
electrolytic water to the cathodic electrolytic water communicating passage 60
only, so that the cathodic electrolytic water produced in the primary
electrolyzer 10 can be routed through the cathodic electrolytic water
communicating passage 60 and through the primary cathodic electrolytic water
outlet 62. The fourth switching valve 50 is switched so as to communicate the
secondary electrolyzer 30 with the atmospheric side of the secondary anodic
electrolytic water outlet 48. The fifth switching valve 58 is switched so as to
communicate the secondary electrolyzer 30 with the atmospheric side of the
secondary cathodic electrolytic water outlet 56.
The cathodic
electrolytic water removed through the primary cathodic electrolytic water
outlet 62 is an electrolytic alkaline water, and this cathodic electrolytic
water is used in the conventional manner. However, the anodic electrolytic water
that is conventionally disposed of as waste is electrolyzed again in the
secondary electrolyzer 30.
The foregoing also applies to a case wherein
the anodic electrolytic water is produced as the major product and is
electrolyzed in the secondary electrolyzer 30. In this case, although the
cathodic electrolytic water by-product is conventionally disposed of as waste,
it too may be electrolyzed in another secondary electrolyzer (not illustrated).
When a water is electrolyzed in the primary electrolyzer 10 and the
secondary electrolyzer 30 with the switching valves 24, 26, 34, 50, and 58
switched as mentioned above, the anodic electrolytic water produced in the
primary electrolyzer 10 is introduced into the secondary electrolyzer 30 from
the first switching valve 24 through the secondary inlet 32 to be electrolyzed
in the secondary electrolyzer 30. The secondary anodic electrolytic water
electrolyzed in the anode compartment of the secondary electrolyzer 30 is
removed through the secondary anodic electrolytic water outlet 48 and through
the fourth switching valve 50. On the other hand, the cathodic electrolytic
water produced in the cathode compartment of the secondary electrolyzer 30, by
electrolysis of the anodic electrolytic water from the primary electrolyzer 10,
is removed through the secondary cathodic electrolytic water outlet 56 and
through the fifth switching valve 58.
Furthermore, the fourth switching
valve 50 may be switched to connect the secondary anodic electrolytic water
outlet 48, leading from the secondary electrolyzer 30, to the anodic
electrolytic water communicating passage 52, 50 that the first switching valve
24 can be connected not only to the combined outlet 28 but also to the anodic
electrolytic water communicating passage 52. In this case, the anodic
electrolytic water produced in the primary electrolyzer 10 and the anodic
electrolytic water produced in the secondary electrolyzer 30 are mixed, and the
mixed anodic electrolytic water is removed through the primary anodic
electrolytic water outlet 54.
Next, the operation of the apparatus will
be described for the case where only the cathodic electrolytic water produced in
the primary electrolyzer 10 is electrolyzed in the secondary electrolyzer 30.
The second switching valve 26 is switched to connect the outgoing passage 22 for
the primary cathodic electrolytic water to the combined outlet 28, so that the
cathodic electrolytic water produced in the primary electrolyzer 10 can be
introduced into the secondary electrolyzer 30 from the combined outlet passage
28 through the secondary inlet 32. The third switching valve 34 provided in the
secondary inlet 32 is switched so as to connect the combined outlet 28 to the
bypass 40. That is, the cathodic electrolytic water produced in the primary
electrolyzer 10 is routed so as not to pass through the chlorine remover 36.
The first switching valve 24 is switched to connect the outlet 20 for
the primary anodic electrolytic water to the anodic electrolytic water
communicating passage 52 only, so that the anodic electrolytic water produced in
the primary electrolyzer 10 can be removed through the anodic electrolytic water
communicating passage 52 and through the primary anodic electrolytic water
outlet 54. The fifth switching valve 58 is switched so as to communicate the
secondary electrolyzer 30 with the atmospheric side of the secondary cathodic
electrolytic water outlet 56. The fourth switching valve 50 is switched so as to
communicate the secondary electrolyzer 30 with the atmospheric side of the
secondary anodic electrolytic water outlet 48.
The anodic electrolytic
water removed through the primary anodic electrolytic water passage 54 is an
electrolytic acid water, and this anodic electrolytic water is put to
conventional uses. However, the cathodic electrolytic water that has
conventionally been disposed of as waste is electrolyzed again in the secondary
electrolyzer 30.
When cathodic electrolytic water which is produced as
the major product is again electrolyzed in the secondary electrolyzer 30,
although the anodic electrolytic water taken would conventionally be disposed as
waste, it may be electrolyzed in another secondary electrolyzer (not
illustrated).
When a water is electrolyzed in the primary electrolyzer
10 and the secondary electrolyzer 30 with the switching valves 24, 26, 34, 50,
and 58 positioned as mentioned above, the cathodic electrolytic water produced
in the primary electrolyzer 10 is introduced into the secondary electrolyzer 30
from the second switching valve 26 through the secondary inlet 32 to be
electrolyzed in the secondary electrolyzer 30. The secondary cathodic
electrolytic water produced in the cathode area of the secondary electrolyzer 30
is removed through the secondary cathodic electrolytic water outlet 56 via the
fifth switching valve 58. On the other hand, the water produced in the anode
area of the secondary electrolyzer 30 from the cathodic electrolytic water
produced in the primary electrolyzer 10 is removed through the secondary anodic
electrolytic water outlet 48 via the fourth switching valve 50.
The
fifth switching valve 58 may be switched to connect the secondary cathodic
electrolytic water outlet 56 on the side of the secondary electrolyzer 30 to the
cathodic electrolytic water communicating passage 60, 50 that the second
switching valve 26 can be connected not only to the combined outlet 28 but also
to the cathodic electrolytic water communicating passage 60. In this
arrangement, the cathodic electrolytic water produced in the primary
electrolyzer 10 and the cathodic electrolytic water electrolyzed in the
secondary electrolyzer 30 are mixed, and the mixed cathodic electrolytic water
is removed through the primary cathodic electrolytic water outlet 62.
In
another embodiment, a mixture of the anodic electrolytic water and the cathodic
electrolytic water produce d in the primary electrolyzer 10 is electrolyzed in
the secondary electrolyzer 30. In this case, the first switching valve 24 is
switched to connect the outlet 20 for the primary anodic electrolytic water to
the combined outlet 28, and the second switching valve 26 is switched to connect
the outlet 22 for the primary cathodic electrolytic water to the combined outlet
28. Thus, the anodic electrolytic water and the cathodic electrolytic water
produced in the primary electrolyzer 10 are mixed in the combined outlet 28, and
this water mixture is routed to the secondary electrolyzer 30 through the
secondary inlet passage 32. The third switching valve 34 provided in the
secondary inlet 32 is switched so as to communicate the combined outlet 28 with
the bypass 40. That is, the cathodic electrolytic water produced in the primary
electrolyzer 10 is routed so as not to pass through the chlorine remover 36.
The fourth switching valve 50 is switched to bring the secondary
electrolyzer 30 into communication with the atmospheric side of the secondary
anodic electrolytic water outlet 48. The fifth switching valve 58 is switched so
as to bring the secondary electrolyzer 30 into communication with the
atmospheric side of the secondary cathodic electrolytic water outlet 56.
Water is electrolyzed in the primary electrolyzer 10 and in the
secondary electrolyzer 30 with the switching valves 24, 26, 34, 50, and 58
positioned as mentioned above. As the result, the anodic electrolytic water and
the cathodic electrolytic water electrolyzed in the primary electrolyzer 10 are
mixed and introduced into the secondary electrolyzer 30 through the third
switching valve 34 and the secondary inlet 32 to be electrolyzed in the
secondary electrolyzer 30. The anodic electrolytic water produced in the anode
area of the secondary electrolyzer 30 is removed via the secondary anodic
electrolytic water outlet 48 via the fourth switching valve 50. The cathodic
electrolytic water produced in the cathode area of the secondary electrolyzer 30
is removed through the secondary cathodic electrolytic water outlet 56 via the
fifth switching valve 58.
The fourth switching valve 50 may be switched
to connect the secondary anodic electrolytic water passage 48 to the anodic
electrolytic water communicating passage 52, so that the first switching valve
24 can be connected not only to the combined outlet 28 but also to the anodic
electrolytic water communicating passage 52. Moreover, the fifth switching valve
58 may be switched to connect the secondary cathodic electrolytic water outlet
56 to the cathodic electrolytic water communicating passage 60, so that the
second switching valve 26 can be connected not only to the combined outlet 28
but also to the cathodic electrolytic water communicating passage 60.
Experiment #1
In experiment 1 a service water is electrolyzed in
the primary electrolyzer 10 and the primary electrolytic water is secondarily
electrolyzed in the secondary electrolyzer 30.
The service water used in
this experiment showed pH 7.6, electric conductivity 160 .mu.S/cm, dissolved
oxygen concentration 8.5 mg/l, oxidation reduction potential 584 mv, and free
dissolved chlorine concentration 0.6 mg/l. The primary electrolyzer 10 was
operated with a water temperature of 21.6.degree. C., water flow rate of 1.41
1/mm, and electrolyzing voltage at 18 V. As the result, a primary anodic
electrolytic water was produced which showed a pH of 4.5, an electric
conductivity of 189 .mu.S/cm, a dissolved oxygen concentration of 10.5 mg/l, an
oxidation reduction potential of 780 mv, a free dissolved chlorine concentration
of 1.3 mg/l, and a primary cathodic electrolytic water was produced which showed
a pH of 9.6, an electric conductivity of 210 .mu.S/cm, a dissolved oxygen
concentration of 6.8 mg/l, an oxidation reduction potential of -153 mv, and a
free dissolved chlorine concentration of 0.2 mg/l.
Only the primary
anodic electrolytic water produced in the primary electrolyzer 10 was
secondarily electrolyzed in the secondary electrolyzer 30, and a secondary
anodic electrolytic water was produced which showed a pH of 3.2, an electric
conductivity of 381 .mu.S/cm, a dissolved oxygen concentration of 14.1 mg/l, an
oxidation reduction potential of 930 mv, a free dissolved chlorine concentration
of 2 mg/l, and a secondary cathodic electrolytic water was produced which showed
a pH of 6.7, an electric conductivity of 141 .mu.S/cm, a dissolved oxygen
concentration of 8.2 mg/l, an oxidation reduction potential of 2 mv, and a free
dissolved chlorine concentration of 0.5 mg/l.
In the secondary anodic
electrolytic water produced by electrolyzing the primary anodic electrolytic
water again in the secondary electrolyzer 30, the dissolved oxygen concentration
increased from 10.5 mg/l to 14.1 mg/l, the oxidation reduction potential changed
from 780 mv to 930 mv, and the free dissolved chlorine concentration changed
from 1.3 mg/l to 2 mg/l. That is, the secondary anodic electrolytic water showed
a higher dissolved oxygen concentration, oxidation reduction potential, and free
dissolved chlorine concentration than the primary anodic electrolytic water.
Therefore, the secondary anodic electrolytic water can be used for its
astringent effect and curative effect in treatment of atopic dermatitis.
Further, the cathodic electrolytic water produced by secondarily
electrolyzing the primary anodic electrolytic water showed a lower oxidation
reduction potential and free dissolved chlorine concentration than the service
water, indicating a suitability for drinking.
On the other hand, when
only the primary cathodic electrolytic water produced in the primary
electrolyzer 10 was secondarily electrolyzed in the secondary electrolyzer 30,
the secondary anodic electrolytic water so produced showed a pH of 8.3, an
electric conductivity of 158 .mu.S/cm, a dissolved oxygen concentration of 9.8
mg/l, an oxidation reduction potential of 38 mv, and a free dissolved chlorine
concentration of 0.9 mg/l. The secondary cathodic electrolytic water so produced
showed a pH of 10.1, an electric conductivity of 312 .mu.S/cm, a dissolved
oxygen concentration of 4.8 mg/l, an oxidation reduction potential of -828 mv,
and a free dissolved chlorine concentration of 0.1 mg/l.
Thus, in the
secondary cathodic electrolytic water produced by electrolyzing the primary
cathodic electrolytic water again in the secondary electrolyzer 30, the
dissolved oxygen concentration decreased from 6.8 mg/l to 4.8 mg/l and the
oxidation reduction potential decreased from -153 mv to -828 mv. Therefore, the
secondary cathodic electrolytic water is preferable to the primary cathodic
electrolytic water for drinking.
The anodic electrolytic water produced
by secondarily electrolyzing the primary cathodic electrolytic water showed a pH
value of 8.3, indicating a slight alkalinity. However, the free dissolved
chlorine concentration was 0.9 mg/l (the average free dissolved chlorine
concentration of the service water is 1.0 mg/1), indicating suitability for
general use as service water.
When a mixture of the primary anodic
electrolytic water and primary cathodic electrolytic water produced in the
primary electrolyzer 10 was secondarily electrolyzed in the secondary
electrolyzer 30, an anodic electrolytic water was produced which showed a pH of
3.84, an electric conductivity of 230 .mu.S/cm, a dissolved oxygen concentration
of 12.6 mg/l, an oxidation reduction potential of 900 mv, and a free dissolved
chlorine concentration of 2 mg/l. A cathodic electrolytic water was produced
which showed a pH of 10.6, an electric conductivity of 250 .mu.S/cm, a dissolved
oxygen concentration of 5.6 mg/l, an oxidation reduction potential of -460 mv,
and a free dissolved chlorine concentration of 0.2 mg/l.
Thus,
secondarily electrolyzing the mixture of the primary anodic electrolytic water
and primary cathodic electrolytic water will raise the dissolved oxygen
concentration of 10.5 mg/l and oxidation reduction potential of 780 mv of the
primary anodic electrolytic water to higher levels, i.e. to a dissolved oxygen
concentration of 12.6 mg/l and an oxidation reduction potential of 900 mv in the
secondary anodic electrolytic water, thus improving the quality of water as
compared to the primary electrolytic acid water. Furthermore, the secondary
electrolysis lowers the dissolved oxygen concentration of 6.8 mg/l and oxidation
reduction potential of -153 mv of the primary cathodic electrolytic water, i.e.
to a dissolved oxygen concentration of 5.6 mg/l and an oxidation reduction
potential of -460 mv in the secondary cathodic electrolytic water, thus
improving the quality of the water as compared to the primary electrolytic
alkaline water.
Experiment #2
In experiment 2 service water of
the same quality as in experiment 1 was electrolyzed with the same water flow
rate as in experiment 1 and with an electrolyzing voltage of 28 V. As a result,
a primary anodic electrolytic water was produced which showed a pH of 3.52, an
electric conductivity of 389 .mu.S/cm, a dissolved oxygen concentration of 12.4
mg/l, an oxidation reduction potential of 820 mv, and a free dissolved chlorine
concentration of 1.5 mg/l. Further, a primary cathodic electrolytic water was
produced which showed a pH of 10.6, an electric conductivity of 313 .mu.S/cm, a
dissolved oxygen concentration of 6.8 mg/l, an oxidation reduction potential of
-758 mv, and a free dissolved chlorine concentration of 0.3 mg/l.
Next,
only the primary anodic electrolytic water produced in the primary electrolyzer
10 was secondarily electrolyzed in the secondary electrolyzer 30, and a
secondary anodic electrolytic water was produced which showed a pH of 2.7, an
electric conductivity of 940 .mu.S/cm, a dissolved oxygen concentration of 22.5
mg/l, an oxidation reduction potential of 1030 mv, and a free dissolved chlorine
concentration of 10 mg/l. This secondary anodic electrolytic water had a high
free dissolved chlorine concentration as compared to the free dissolved chlorine
concentration obtained in experiment 1 (2 mg/l), indicating a strong
bactericidal activity.
In addition, a cathodic electrolytic water was
produced which showed a pH of 9.4, an electric conductivity of 101 .mu.S/cm, a
dissolved oxygen concentration of 6.2 mg/l, an oxidation reduction potential of
-825 mv, and a free dissolved chlorine concentration of 0.4 mg/l. Although this
cathodic electrolytic water showed a slightly high free dissolved chlorine
concentration, it is still within the standard and can be an ideal drinking
water.
When only the primary cathodic electrolytic water produced in the
primary electrolyzer 10 was secondarily electrolyzed in the secondary
electrolyzer 30, an anodic electrolytic water was produced which showed a pH of
7.1, an electric conductivity of 72 .mu.S/cm, a dissolved oxygen concentration
of 21.9 mg/l, an oxidation reduction potential of 671 mv, and a free dissolved
chlorine concentration of 8 mg/l. This anodic electrolytic water was neutral and
very high in dissolved oxygen concentration (double that of a conventional
oxygen saturated water), and can be used as a bacteriostatic agent.
The
secondary cathodic electrolytic water thus produced showed a pH of 11.4, an
electric conductivity of 521 .mu.S/cm, a dissolved oxygen concentration of 4.2
mg/l, an oxidation reduction potential of -863 mv, and a free dissolved chlorine
concentration of 0.01 mg/l. This secondary cathodic electrolytic water shows a
high pH value. However, it contains a slight amount of metal ions (sodium ion,
potassium ion, etc.) paired with the hydroxyl groups and, therefore, the
cathodic electrolytic water is unstable in pH, easy to drink without a sense of
rejection, and can be an anoxic water that does not cause a disorder due to
strong alkalinity.
When the anodic electrolytic water and cathodic
electrolytic water produced in the primary electrolyzer 10 were mixed, and the
mixed water showed a pH of 10.62, an electric conductivity of 207 .mu.S/cm, a
dissolved oxygen concentration of 7.8 mg/l, an oxidation reduction potential of
-97 mv, a free dissolved chlorine concentration 0.66 mg/l, which is a weak
alkaline water with an oxidation reduction potential significantly lower than
the raw water. This mixed water was secondary electrolyzed in the secondary
electrolyzer 30, and an anodic electrolytic water was produced which showed a pH
of 3.1, an electric conductivity of 402 .mu.S/cm, a dissolved oxygen
concentration of 26.7 mg/l, an oxidation reduction potential of 950 mv, and a
free dissolved chlorine concentration of 7.5 mg/l. Thus, the dissolved oxygen
concentration and oxidation reduction potential of the secondary anodic
electrolytic water were higher than in the primary anodic electrolytic water,
and thus the quality of the secondary anodic electrolytic water was higher than
that of the primary electrolytic acid water.
The secondary cathodic
electrolytic water produced from the mixed water showed a pH of 11.2, an
electric conductivity of 353 .mu.S/cm, a dissolved oxygen concentration of 5
mg/l, an oxidation reduction potential of -844 mv, and a free dissolved chlorine
concentration of 0.5 mg/l. This secondary cathodic electrolytic water is
effective for sterilization and oxidation and is an electrolytic alkaline water
with a strong reduction power. Thus, the dissolved oxygen concentration and
oxidation reduction potential of the secondary cathodic electrolytic water are
lower than in the primary cathodic electrolytic water, and its quality is better
than that of the primary electrolytic alkaline water.
In order to remove
the dissolved chlorine from the electrolytic alkaline water, the water was
passed through an activated carbon filter, and the result was that, although the
pH value decreased 0.2 to 0.5, the electric conductivity and the dissolved
oxygen concentration were almost unchanged, and the oxidation reduction
potential increased by 50 to 100 mv, all of which values are within ranges
effective for drinking.
Next, the method of removing chlorine contained
in the water primarily electrolyzed, prior to secondary electrolysis, will be
described. In FIG. 1, first, the third switching valve 34 provided in the
secondary inlet 32 is switched so as to pass the water from the secondary inlet
32 in sequence through the chlorine remover 36 and the check-valve 38.
The electrolytic water produced in the primary electrolyzer 10 is passed
through the chlorine remover 36 containing a chlorine removal filter of an
activated carbon or the like. After the free dissolved chlorine is removed, the
water is electrolyzed in the second electrolyzer 30. When the primary anodic
electrolytic water was electrolyzed in the electrolyzer 30 and the secondary
anodic electrolytic water so produced with chlorine filtering was compared to
that produced without chlorine filtering, the pH value was seen to be higher by
0.3, the electric conductivity lower by about 200 .mu.S/cm, the oxidation
reduction potential lower by about 100 mv, and the dissolved oxygen
concentration and free dissolved chlorine concentration did not change. And, in
the cathode area, the pH value decreased by about 1, the electric conductivity
increased by about 30 .mu.S/cm, the oxidation reduction potential decreased by
about 100 mv, and the free dissolved chlorine concentration decreased to 0.1.
When the secondary cathodic electrolytic water produced with chlorine
filtering was compared with that produced without chlorine filtering, the pH
value was seen to be lower by about 3 and the neutral water changed into a
distinctively acidic water, the electric conductivity increased by about 10
.mu.S/cm, the oxidation reduction potential increased by about 100 mv, and the
dissolved oxygen concentration and the free dissolved chlorine concentration did
not change. In the cathode area, the pH value did not change, the electric
conductivity decreased by about 50 .mu.S/cm, and the oxidation reduction
potential and free dissolved chlorine concentration decreased to 0.1.
Consequently, secondarily electrolyzing the primary electrolytic water
after filtering out the chlorine contained therein tends to increase the pH and
to decrease the oxidation reduction potential in the secondary anodic
electrolytic water; however, the value remains within a range showing the
astringent effect, and the secondary cathodic electrolytic water shows a
decrease in the dissolved chlorine so that it becomes an electrolytic alkaline
water more suitable for drinking.
Thus, by chlorine filtering between
the primary and secondary electrolysis, the free dissolved chlorine
concentration in the secondary electrolytic water is decreased to less than 15
ppm.
Experiment #3
In the forgoing embodiment, secondary
electrolysis was described; however, when a tertiary electrolysis or still
higher-order electrolysis is performed, a higher-order electrolytic anodic water
shows a higher dissolved oxygen concentration and oxidation reduction potential,
and a higher-order cathodic electrolytic water shows a lower dissolved oxygen
concentration and oxidation reduction potential.
In a third experiment
tertiary electrolysis was performed, that is, the tertiary electrolysis was
applied to the waters obtained through the secondary electrolysis. Among the
tertiary electrolytic waters, the tertiary anodic electrolytic water produced by
electrolyzing a secondary anodic electrolytic water, in turn produced by
electrolyzing the primary anodic electrolytic water, was found to display a
distinctive sterilization effect.
The experimental data for the service
water (raw water), the primary anodic electrolytic water, the secondary anodic
electrolytic water, and the tertiary anodic electrolytic water was as follows.
______________________________________
oxidation
dissolved
free dissolved
reduction
oxygen chlorine
pH potential
concentration
concentration
______________________________________
service water
7.19 574 9.6 0.6
primary a.w.
2.56 871 11.6 6
secondary a.w.
1.87 907 16.4 6
tertiary a.w.
1.45 1071 24.6 15
______________________________________
"a.w." is an abbreviation for anodic ectrolytic water.
From the results of this experiment, a big change is seen in the
pH between the primary anodic electrolytic water and the secondary anodic
electrolytic water, i.e. 2.56 versus 1.87, which is a big change, while the
oxidation reduction potential and dissolved oxygen concentration changed only
slightly, and the free dissolved chlorine concentration remained unchanged. In
contrast, between the secondary and the tertiary anodic electrolytic water, the
pH decreased to less than 1.5 (the acidity became very strong) and, furthermore,
the oxidation reduction potential increased by about 160 mv, the dissolved
oxygen concentration increased to about 1.5 times, and the free dissolved
chlorine concentration increased 2.5 times.
Thus, when the secondary
anodic electrolytic water is electrolyzed to produce the tertiary anodic
electrolytic water, the pH, oxidation reduction potential, dissolved oxygen
concentration, and free dissolved chlorine concentration are significantly
changed to values exhibiting a bactericidal effect, and the tertiary anodic
electrolytic water displays a very strong bactericidal activity that had not
been obtained up to the present.
As described hereinabove, according to
the present invention, an electrolytic water in compliance with its intended use
can be transformed into an electrolytic water more effectively used by repeating
electrolysis more than two times, and the electrolytic acid water for drinking
or the electrolytic alkaline water for sterilization, which have conventionally
been disposed of as having virtually no use, can be transformed into
electrolytic waters which can be effectively used.
Furthermore, since
the chlorine contained in the primary electrolytic water is filtered out before
the secondary electrolysis, a special step for the electrolytes and the like
becomes unnecessary; and therefore, an electrolytic water can be obtained easily
and reliably which has an oxidation reduction potential and a dissolved oxygen
concentration in compliance with its intended use and a dissolved chlorine
concentration no higher than needed.
Moreover, the tertiary anodic
electrolytic water possesses significantly better values for pH, oxidation
reduction potential, dissolved oxygen concentration, and free dissolved chlorine
concentration which show a bactericidal effect, and a water can be produced
which has a much stronger bactericidal activity than that previously obtained.
While specific embodiments of the present invention have been
illustrated and described herein, it is realized that numerous modifications and
changes will occur to those skilled in the art. It is therefore to be understood
that the appended claims are intended to cover all such modifications and
changes as fall within the true spirit and scope of the invention.
* * * * *
![[US Patent & Trademark Office, Patent Full Text and Image Database]](United States Patent 5,858,202.files/patfthdr.gif)
![[Home]](United States Patent 5,858,202.files/home.gif)
![[Boolean Search]](United States Patent 5,858,202.files/boolean.gif)
![[Manual Search]](United States Patent 5,858,202.files/manual.gif)
![[Number Search]](United States Patent 5,858,202.files/number.gif)
![[Help]](United States Patent 5,858,202.files/help.gif)
![[Bottom]](United States Patent 5,858,202.files/bottom.gif)
![[View Shopping Cart]](United States Patent 5,858,202.files/cart.gif)
![[Add to Shopping Cart]](United States Patent 5,858,202.files/order.gif)
![[Image]](United States Patent 5,858,202.files/image.gif)
![[US Patent & Trademark Office, Patent Full Text and Image Database]](United States Patent 5,858,202.files/patfthdr.gif)
![[Home]](United States Patent 5,858,202.files/home.gif)
![[Boolean Search]](United States Patent 5,858,202.files/boolean.gif)
![[Manual Search]](United States Patent 5,858,202.files/manual.gif)
![[Number Search]](United States Patent 5,858,202.files/number.gif)
![[Help]](United States Patent 5,858,202.files/help.gif)
![[Bottom]](United States Patent 5,858,202.files/bottom.gif)
![[View Shopping Cart]](United States Patent 5,858,202.files/cart.gif)
![[Add to Shopping Cart]](United States Patent 5,858,202.files/order.gif)
![[Image]](United States Patent 5,858,202.files/image.gif)

